辛可卡因 | 85-79-0
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:64°
-
沸点:478.73°C (rough estimate)
-
密度:1.1145 (rough estimate)
-
溶解度:DMSO:45.0(最大浓度 mg/mL);131.02(最大浓度 mM)
-
LogP:4.4 at 25℃ and pH7
-
物理描述:Solid
-
颜色/状态:Colorless or almost colorless powder
-
气味:Odorless
-
水溶性:-3.7
-
稳定性/保质期:
SENSITIVE TO LIGHT
-
分解:When heated to decomposition it emits toxic fumes of nitroxides.
-
碰撞截面:196.2 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)]
-
保留指数:2706;2677;2693;2693;2690;2740;2675;2701
计算性质
-
辛醇/水分配系数(LogP):4.4
-
重原子数:25
-
可旋转键数:10
-
环数:2.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:54.5
-
氢给体数:1
-
氢受体数:4
ADMET
安全信息
-
危险品标志:Xn
-
安全说明:S26,S39
-
危险类别码:R22
-
RTECS号:GD3150000
-
海关编码:2933499090
-
危险性防范说明:P280,P305+P351+P338,P310
-
危险性描述:H302,H315,H319,H332,H335
SDS
Section 1. Chemical Product and Company Identification
Dibucaine
Common Name/
Trade Name
Dibucaine
Section 3. Hazards Identification
Potential Acute Health Hazardous in case of ingestion. Slightly hazardous in case of skin contact (irritant), of eye contact (irritant),
Effects of inhalation. Severe over-exposure can result in death.
CARCINOGENIC EFFECTS: Not available.
Potential Chronic Health
MUTAGENIC EFFECTS: Not available.
Effects
TERATOGENIC EFFECTS: Not available.
DEVELOPMENTAL TOXICITY: Not available.
The substance may be toxic to cardiovascular system, central nervous system (CNS).
Repeated or prolonged exposure to the substance can produce target organs damage. Repeated exposure
to a highly toxic material may produce general deterioration of health by an accumulation in one or many
human organs.
Section 4. First Aid Measures
Eye Contact Check for and remove any contact lenses. In case of contact, immediately flush eyes with plenty of water
for at least 15 minutes. Get medical attention if irritation occurs.
Skin Contact In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing
contaminated clothing and shoes. Cover the irritated skin with an emollient. Wash clothing before reuse.
Thoroughly clean shoes before reuse. Get medical attention immediately.
Serious Skin Contact Not available.
Inhalation If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen.
Get medical attention immediately.
Serious Inhalation Evacuate the victim to a safe area as soon as possible. Loosen tight clothing such as a collar, tie, belt or
waistband. If breathing is difficult, administer oxygen. If the victim is not breathing, perform mouth-to-mouth
resuscitation. WARNING: It may be hazardous to the person providing aid to give mouth-to-mouth
resuscitation when the inhaled material is toxic, infectious or corrosive. Seek immediate medical attention.
Ingestion If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by
mouth to an unconscious person. Loosen tight clothing such as a collar, tie, belt or waistband. Get medical
attention immediately.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Flash Points Not available.
Flammable Limits Not available.
Products of Combustion These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...).
Fire Hazards in Presence of Slightly flammable to flammable in presence of heat.
Various Substances
Explosion Hazards in Risks of explosion of the product in presence of mechanical impact: Not available.
Presence of Various Risks of explosion of the product in presence of static discharge: Not available.
Substances
Fire Fighting Media SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
Special Remarks on When heated to decomposition it emits toxic fumes of nitroxides.
Fire Hazards
Special Remarks on Explosion Not available.
Hazards
Dibucaine
Section 6. Accidental Release Measures
Small Spill Use appropriate tools to put the spilled solid in a convenient waste disposal container.
Large Spill Poisonous solid.
Stop leak if without risk. Do not get water inside container. Do not touch spilled material. Use water spray
to reduce vapors. Prevent entry into sewers, basements or confined areas; dike if needed. Eliminate all
Section 7. Handling and Storage
Keep away from heat. Keep away from sources of ignition. Ground all equipment containing material. Do
Precautions
not ingest. Do not breathe dust. Wear suitable protective clothing. If ingested, seek medical advice
immediately and show the container or the label.
Storage Keep container tightly closed. Keep container in a cool, well-ventilated area.
Section 8. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels
below recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep
exposure to airborne contaminants below the exposure limit.
Personal Protection Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent.
Gloves.
Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be
a Large Spill used to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a
specialist BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Solid. (Hygroscopic powder.) Odor Odorless.
Not available.
Taste
343.46 g/mole
Molecular Weight
Color White.
Not applicable.
pH (1% soln/water)
Boiling Point Not available.
Melting Point 62°C (143.6°F) - 65 C.
Critical Temperature Not available.
Specific Gravity Not available.
Not applicable.
Vapor Pressure
Vapor Density 13.1 (Air = 1)
Not available.
Volatility
Odor Threshold Not available.
Water/Oil Dist. Coeff. Not available.
Not available.
Ionicity (in Water)
Dispersion Properties See solubility in water, diethyl ether.
Soluble in diethyl ether.
Solubility
Insoluble in cold water, hot water.
Soluble in Ethanol, Chloroform, Petroleum Ether
Dibucaine
Section 10. Stability and Reactivity Data
The product is stable.
Stability
Instability Temperature Not available.
Excess heat
Conditions of Instability
Incompatibility with various Not available.
substances
Non-corrosive in presence of glass.
Corrosivity
Special Remarks on Sensitive to light.
Reactivity
Special Remarks on Not available.
Corrosivity
Will not occur.
Polymerization
Section 11. Toxicological Information
Routes of Entry Inhalation. Ingestion.
Toxicity to Animals LD50: Not available.
LC50: Not available.
Chronic Effects on Humans May cause damage to the following organs: cardiovascular system, central nervous system (CNS).
Other Toxic Effects on Hazardous in case of ingestion.
Humans Slightly hazardous in case of skin contact (irritant), of inhalation.
Special Remarks on Not available.
Toxicity to Animals
Special Remarks on Not available.
Chronic Effects on Humans
Special Remarks on other Acute Potential Health Effects:
Toxic Effects on Humans Skin: May cause skin irritation. Can cause local numbness.
Eyes: May cause eye irritation.
Inhalation: May cause respiratory tract irritation.
Ingestion: Harmful if swallowed. Can cause vomiting, pallor, increased sweating, blurred or double vision.
May affect behavior/'central nervous system (headache, staggering, convulsions, dizziness, confusion,
drowsiness, trembling, shivering, anxiety, tiredness, weakness, excitement, somnolence), cardiovascular
system (low blood pressure, irregular heartbeat, cardiac arresst). May also cause ringning or buzzing in the
ears, feeling hot or cold, or numb.
Medical Conditions Aggravated by Exposure: hypersensitivity to material; hear disease; chronic obstructive
lung disease.
Section 12. Ecological Information
Ecotoxicity Not available.
BOD5 and COD Not available.
Possibly hazardous short term degradation products are not likely. However, long term degradation
Products of Biodegradation
products may arise.
The products of degradation are less toxic than the product itself.
Toxicity of the Products
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Dibucaine
Section 13. Disposal Considerations
Waste Disposal Waste must be disposed of in accordance with federal, state and local environmental
control regulations.
Section 14. Transport Information
DOT Classification CLASS 6.1: Poisonous material.
UNNA: 1544 : Alkaloid, solid, n.o.s. (Dibucaine) PG: III
Identification
Not available.
Special Provisions for
Transport
DOT (Pictograms)
Section 15. Other Regulatory Information and Pictograms
No products were found.
Federal and State
Regulations
California California prop. 65: This product contains the following ingredients for which the State of California has
Proposition 65 found to cause cancer which would require a warning under the statute: No products were found.
Warnings
California prop. 65: This product contains the following ingredients for which the State of California has
found to cause birth defects which would require a warning under the statute: No products were found.
OSHA: Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200).
Other Regulations
EINECS: This product is on the European Inventory of Existing Commercial Chemical Substances (EINECS
no. 201-632-1)
Canada: Not listed on Canadian Domestic Substance List (DSL) or Canadian Non- Domestic Substance
List (NDSL).
China: Not listed on National Inventory.
Japan: Not listed on National Inventory (ENCS).
Korea: Not listed on National Inventory (KECI).
Philippines: Not listed on National Inventory (PICCS).
Australia: Listed on AICS.
WHMIS (Canada) CLASS D-1B: Material causing immediate and serious toxic effects (TOXIC).
Other Classifications
DSCL (EEC) This product is not classified Not applicable.
according to the EU regulations.
Health Hazard
HMIS (U.S.A.) 2 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
2 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
Dibucaine
TDG (Canada)
(Pictograms)
6
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or
equivalent. Wear appropriate respirator
when ventilation is inadequate.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
辛可卡因常用其盐酸盐,为白色结晶粉末,味略苦,熔点95~100℃,易溶于水。
简介辛可卡因又名可卡因、地布卡因、纽白卡因、沙夫卡因、盐酸辛可卡因。化学结构虽有酰胺存在,但仍和酯族局麻药一样,血浆胆碱酯酶能予以水解,临床上可用以鉴定该酯酶的效应。其局麻效能较普鲁卡因大22~25倍,持续时间长,但毒性比普鲁卡因大15—20倍,一旦出现中毒,抢救需4—8小时才能逆转。在组织中远较普鲁卡因稳定,因此麻醉作用持续时间较久(约为普鲁卡因的3倍)。适用于硬膜外麻醉以及腰麻,容易通过粘膜,也用于表面麻醉;但因其毒性过大(约比普鲁卡因高15倍),较少用于浸润麻醉。
用途辛可卡因主要适用于硬膜外麻醉以及腰麻。本品容易通过粘膜,也用于表面麻醉;但因其毒性过大(约比普鲁卡因高15倍),较少用于浸润麻醉。
制备-
2-氯-N-[2-(二乙基氨基)乙基]-4-喹啉甲酰胺的合成
室温下向3000ml三口瓶中加入2-羟基-4-喹啉羧酸200g,甲苯1500ml搅拌下滴加氯化亚砜158g,升温至75℃反应2小时。降温至25℃后减压浓缩,再加500ml甲苯继续减压浓缩至干。直接加入2000ml甲苯稀释后加入到5000ml三口瓶中,再加入N,N-二乙基二乙胺100g,升温70℃搅拌反应完全后降至室温,加水搅拌30分钟,分液。有机层用水洗两次、饱和食盐水洗涤一次,无水硫酸钠干燥,过滤得2-氯-N-[2-(二乙基氨基)乙基]-4-喹啉甲酰胺即辛可酰胺274g,收率85%。
-
辛可卡因的合成
将200g辛可酰胺、550ml正丁醇和300g正丁醇钠加入至3000ml反应瓶中,逐渐升温至回流3小时后降温至室温。加入纯化水1000ml搅拌30分钟,静止分层30min,弃去水层。有机层加入无水硫酸钠干燥、过滤,滤液减压浓缩。再加入500ml甲苯并升温至60℃搅拌30min,静置后分层。上层甲苯层降温析晶得辛可卡因精制品146g,收率65%,纯度为99.7%。
Dibucaine (Cinchocaine) 是一种钠通道抑制剂,并且是有效的 SChE 抑制剂。
体外研究-
Dibucaine (Cinchocaine) 减少了 BSA-gold 复合体在寄生虫的储备囊泡中的降解,这并非由整个蛋白水解活性的抑制或 cruzipain 表达水平的降低引起。
-
Dibucaine 是一种四价铵化合物,在2分钟内以可逆方式对 SChE 的抑制作用非常强。它的 IC(50) 值分别为 BuTch 或 AcTch 时的 5.3 μM 和 3.8 μM。相对于 BuTch,其抑制是竞争性的,K(i)为1.3 μM;而相对于 AcTch 的抑制是线性混合型(竞争/非竞争),其 K(i) 和 K(I) 分别为0.66和2.5 μM。Dibucaine 拥有一个但氧基侧链,类似于 BuTch 的丁酰基团,并且比 AcTch 长一个乙二醇基团。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-butoxy-quinoline-4-carboxylic acid amide 98006-39-4 C14H16N2O2 244.293 N-(2-(二乙基)胺乙基)-2-氯基-4-喹啉甲酰胺 2-chloro-quinoline-4-carboxylic acid-(2-diethylamino-ethylamide) 87864-14-0 C16H20ClN3O 305.807 —— 2-(n-butyloxy)-4-(n-butyloxycarbonyl)-quinoline 107779-36-2 C18H23NO3 301.386 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 辛可卡因氮氧化物 dibucaine N-oxide 87864-07-1 C20H29N3O3 359.469 辛可卡因EP杂质C 2-Hydroxy-chinolin-4-carbonsaeure-diethylethylendiamid 87864-08-2 C16H21N3O2 287.362
反应信息
-
作为反应物:参考文献:名称:[EN] FORMULATIONS OF N-OXIDE PRODRUGS OF LOCAL ANESTHETICS FOR THE TREATMENT OF PULMONARY INFLAMMATION ASSOCIATED WITH ASTHMA, BROCHITIS, AND COPD
[FR] PROMEDICAMENTS N-OXYDE D'ANESTHESIQUES LOCAUX DESTINES AU TRAITEMENT DE L'INFLAMMATION PULMONAIRE ASSOCIEE A L'ASTHME, A LA BRONCHITE ET A LA BPCO摘要:本文描述了一种利多卡因和相关局部麻醉剂的前药组成物或配方,可通过喷雾给药。该配方含有有效量的利多卡因N-氧化物前药或局部麻醉N-氧化物前药,能够抑制哮喘肺部的炎症。该N-氧化物前药以5毫升四分之一正常盐水溶液的形式制备,pH值在1.0至7.0之间。该方法用于通过喷雾给药的配方治疗呼吸道炎症,其质量中等平均直径主要在1到5微米之间,由雾化或干粉吸入器产生,并作为单一的N-氧化物前药治疗或与β-激动剂联合使用。公开号:WO2005044233A1 -
作为产物:描述:参考文献:名称:鉴定dibucaine衍生物作为新型有效肠病毒2C解旋酶抑制剂:体外,体内和联合治疗研究。摘要:肠病毒A71(EV-A71)是引起手足口病(HFMD)的人类病原体,严重威胁着婴幼儿的安全和生命。但是,没有获得许可的直接抗病毒药可以治愈手足口病。在这项研究中,开发了一系列喹啉甲酰胺类似物作为有效的肠病毒抑制剂,随后的系统结构活性关系(SAR)研究表明,这些喹啉甲酰胺类似物表现出了良好的治疗EV-A71感染的能力。如上所述,最有效的EV-A71抑制剂6i 在RD细胞中显示出良好的抗EV-A71活性(EC 50 = 1.238μM)。此外,化合物6i可以有效预防6 mg / kg剂量的病毒感染小鼠的死亡。当与依替丁(0.1 mg / kg)联合使用时,这种治疗可以完全预防病毒感染小鼠的临床症状和死亡。机制研究表明,化合物6i通过靶向2C解旋酶抑制EV-A71,从而阻碍RNA重塑和代谢。综上所述,这些数据表明6i是一种有前途的EV-A71抑制剂,作为前导化合物值得进行广泛的临床前研究。DOI:10.1016/j.ejmech.2020.112310
文献信息
-
[EN] THERAPEUTIC ACRYLATES AS ENHANCED MEDICAL ADHESIVES<br/>[FR] ACRYLATES THÉRAPEUTIQUES UTILES EN TANT QU'ADHÉSIFS MÉDICAUX AMÉLIORÉS申请人:UNIV CARNEGIE MELLON公开号:WO2018052936A1公开(公告)日:2018-03-22Provided herein are therapeutic acrylate compounds useful as medical adhesives, comprising a therapeutic agent covalently linked to a methacrylate or cyanoacrylate moiety. Adhesive compositions and kits, such as liquid sutures and bone cement also are provided along with uses for the compositions.
-
[EN] TARGETED DRUG DELIVERY THROUGH AFFINITY BASED LINKERS<br/>[FR] ADMINISTRATION CIBLÉE D'UN MÉDICAMENT FAISANT APPEL À DES COUPLEURS FONDÉS SUR L'AFFINITÉ申请人:INVICTUS ONCOLOGY PVT LTD公开号:WO2015148126A1公开(公告)日:2015-10-01The current invention discloses targeted drug delivery conjugates comprising a targeting moiety linked to a drug via a molecule having an affinity for the targeting moiety. Typically, the conjugate comprises a targeting ligand and a molecule of interest, e.g., a therapeutic agent. The targeting ligand and the molecule of interest are linked to each other via an affinity ligand. The affinity ligand is further covalently or non-covalently linked to a drug or therapeutic agent. The drug can be modified to make it more soluble and so that it cleaves from the linking molecule at the target site.
-
ASYMMETRIC BIFUNCTIONAL SILYL MONOMERS AND PARTICLES THEREOF AS PRODRUGS AND DELIVERY VEHICLES FOR PHARMACEUTICAL, CHEMICAL AND BIOLOGICAL AGENTS申请人:The University of North Carolina at Chapel Hill公开号:US20170021030A1公开(公告)日:2017-01-26Asymmetric bifunctional silyl (ABS) monomers comprising covalently linked pharmaceutical, chemical and biological agents are described. These agents can also be covalently bound via the silyl group to delivery vehicles for delivering the agents to desired targets or areas. Also described are delivery vehicles which contain ABS monomers comprising covalently linked agents and to vehicles that are covalently linked to the ABS monomers. The silyl modifications described herein can modify properties of the agents and vehicles, thereby providing desired solubility, stability, hydrophobicity and targeting.
-
Non-peptidyl inhibitors of VLA-4 dependent cell binding useful in treating inflammatory, autoimmune, and respiratory diseases申请人:——公开号:US20020049236A1公开(公告)日:2002-04-25There is disclosed a genus of non-peptidyl compounds, wherein said compounds are VLA-4 inhibitors useful in treating inflammatory, autoimmune, and respiratory diseases, and wherein said compounds comprise a compound of Formula (1.0.0): 1 and pharmaceutically acceptable salts and other prodrug derivatives thereof, wherein: A is (C 1 -C 6 ) alkyl, cycloalkyl, aryl, heteroaryl or heterocyclyl optionally substituted with 0 to 3 R 9 ; or is a member selected from the group consisting of the following radicals: A 1 -NHC(═O)NH-A 2 -, A 1 -NHC(═O)O-A 2 -, A 1 -OC(═O)NH-A 2 -, A 1 -NHSO 2 NH-A 2 -, A 1 -NHC(═O)-A 2 -, A 1 -C(═O)NH-A 2 -, A 1 -NHSO 2 -A 2 -, A 1 -SO 2 NH-A 2 -, A 1 -(CH 2 ) r -A 2 -, where A 1 and A 2 are each independently selected from the group consisting of hydrogen, aryl, (C 1 -C 6 ) alkyl, (C 2 -C 6 ) alkenyl, (C 2 -C 6 ) alkynyl, cycloalkyl, heteroaryl, and heterocyclyl substituted with 0 to 3 R 9 ; B is a member independently selected from the group consisting of the following: 2 E is a single bond; —O—; —NR 10 —; —CH═CH—; —CC—; —S(═) q ; —CR 11 R 12 NR 10 —; or —CR 11 R 12 ; X is —O—; —C(═O)—; —S(═O) q —; or —NR 10 —; X 1 , X 2 and X 3 are each independently selected from the group consisting of CH, CR 9 or N; Y is a single bond; —C(═O)—; —C(═S)—; or —S(═O) 2 —; R 7 is (C 1 -C 6 ) alkyl; (CH 2 ) k OR 5 ; (CH 2 ) k NR 6 C(═O)R 5 ; (CH 2 ) k NR 6 C(═O)OR 5 ; (CH 2 ) k NR 6 SO 2 R 5 ; (CH 2 ) k NR 6 R 5 ; F; CF 3 ; OCF 3 ; aryl, substituted with 0 to 3 R 9 ; heterocyclyl, substituted with 0 to 3 R 9 ; heteroaryl, substituted with 0 to 3 R 9 ; cycloalkyl, substituted with 0 to 3 R 9 ; or R 7 may be taken together with R 8 to form a cycloalkyl or heterocyclyl ring; or R 7 may be taken together with R 11 to form a cycloalkyl or heterocyclyl ring; and R 8 is hydrogen; F; (C 1 -C 6 ) alkyl or (C 1 -C 6 ) alkoxy.本发明涉及一类非肽类化合物,其中这些化合物是VLA-4抑制剂,用于治疗炎症性、自身免疫和呼吸道疾病,这些化合物包括如下式(1.0.0)的化合物: 1 以及其药学上可接受的盐和其他前药衍生物,其中: A为(C 1 -C 6 )烷基,环烷基,芳基,杂芳基或杂环烷基,可选地取代为0至3个R 9 ;或者是从以下基团中选择的成员:A 1 -NHC(═O)NH-A 2 -,A 1 -NHC(═O)O-A 2 -,A 1 -OC(═O)NH-A 2 -,A 1 -NHSO 2 NH-A 2 -,A 1 -NHC(═O)-A 2 -,A 1 -C(═O)NH-A 2 -,A 1 -NHSO 2 -A 2 -,A 1 -SO 2 NH-A 2 -,A 1 -(CH 2 ) r -A 2 -,其中A 1 和A 2 各自独立地选择自氢,芳基,(C 1 -C 6 )烷基,(C 2 -C 6 )烯基,(C 2 -C 6 )炔基,环烷基,杂芳基和杂环烷基,取代为0至3个R 9 ; B是独立地从以下成员中选择的: 2 E是单键;—O—;—NR 10 —;—CH═CH—;—CC—;—S(═) q ;—CR 11 R 12 NR 10 —;或—CR 11 R 12 ; X是—O—;—C(═O)—;—S(═O) q —;或—NR 10 —;X 1 ,X 2 和X 3 各自独立地选择自CH,CR 9 或N;Y是单键;—C(═O)—;—C(═S)—;或—S(═O) 2 —;R 7 是(C 1 -C 6 )烷基;(CH 2 ) k OR 5 ;(CH 2 ) k NR 6 C(═O)R 5 ;(CH 2 ) k NR 6 C(═O)OR 5 ;(CH 2 ) k NR 6 SO 2 R 5 ;(CH 2 ) k NR 6 R 5 ;F;CF 3 ;OCF 3 ;芳基,取代为0至3个R 9 ;杂环烷基,取代为0至3个R 9 ;杂芳基,取代为0至3个R 9 ;环烷基,取代为0至3个R 9 ;或R 7 可以与R 8 一起形成环烷基或杂环烷基环;或R 7 可以与R 11 一起形成环烷基或杂环烷基环;而R 8 是氢;F;(C 1 -C 6 )烷基或(C 1 -C 6 )烷氧基。
-
MULTIFUNCTIONAL SMALL MOLECULES申请人:Baker, JR. James R.公开号:US20120259114A1公开(公告)日:2012-10-11The present invention relates to dendrimer synthesis. Specifically, the present invention relates to triazine scaffolds capable of click chemistry for one-step synthesis of functionalized dendrimers, and methods of making and using the same.本发明涉及树枝状大分子的合成。具体而言,本发明涉及能够进行点击化学的 三嗪支架,用于一步法合成功能化树枝状大分子,以及制造和使用该支架的方法。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
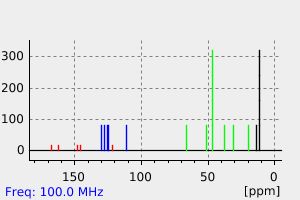
碳谱13CNMR
-
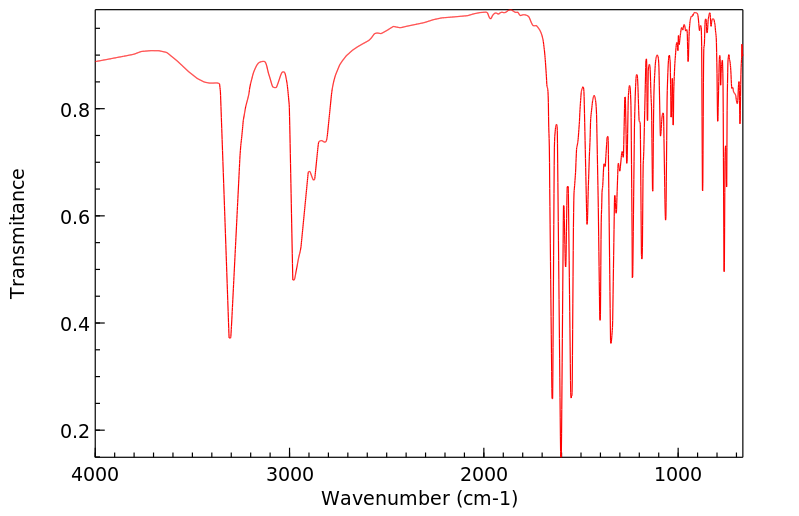
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息