乳酸依沙吖啶 | 1837-57-6
中文名称
乳酸依沙吖啶
中文别名
雷佛奴尔;雷凡醇;6,9-二胺-2-乙氧吖啶;乳酸依沙吖啶(一水物);2-乙氧基-6,9-二氨基吖啶乳酸盐
英文名称
ethacridine lactate
英文别名
rivanol;acrinol;6,9-diamino-2-ethoxyacridine-DL-lactate;2-ethoxy-6,9-diamino-acridine lactate;2-Ethoxy-6,9-diamino-acridinium-lactat;7-ethoxyacridin-10-ium-3,9-diamine;2-hydroxypropanoate
CAS
1837-57-6
化学式
C3H6O3*C15H15N3O
mdl
——
分子量
343.382
InChiKey
IYLLULUTZPKQBW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:235°C
-
沸点:478.66°C (rough estimate)
-
密度:1.2764 (rough estimate)
-
溶解度:DMSO:84.5(最大浓度 mg/mL);246.08(最大浓度 mM)水:69.0(最大浓度 mg/mL);104.84(最大浓度 mM)
-
碰撞截面:156.3 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,没有已知危险反应,避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):2.4
-
重原子数:25
-
可旋转键数:3
-
环数:3.0
-
sp3杂化的碳原子比例:0.22
-
拓扑面积:132
-
氢给体数:4
-
氢受体数:7
安全信息
-
危险品标志:Xn,Xi
-
WGK Germany:3
-
RTECS号:UT6410000
-
海关编码:2933990090
-
安全说明:S24/25,S26,S36/37/39,S37/39
-
危险类别码:R22,R20/21/22,R40,R36/37/38
-
储存条件:请将贮藏器密封,并存放在阴凉、干燥处。确保工作环境有良好的通风或排气设施。
SDS
| Name: | 6 9-Diamino-2-Ethoxyacridine Lactate 95% (Titr.) Material Safety Data Sheet |
| Synonym: | 6,9-Acridinediamine, 2-Ethoxy-, 2-Hydroxypropanote; Acrinol; Ethodin |
| CAS: | 1837-57-6 |
Synonym:6,9-Acridinediamine, 2-Ethoxy-, 2-Hydroxypropanote; Acrinol; Ethodin
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1837-57-6 | Propanoic acid, 2-hydroxy-, compd. wit | 95 | 217-408-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1837-57-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C18H21N3O4.H2O
Molecular Weight: 361.39
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1837-57-6: OD4725000 LD50/LC50:
Not available.
Carcinogenicity:
Propanoic acid, 2-hydroxy-, compd. with7-ethoxy-3,9-acridine - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 1837-57-6: No information available.
Canada
CAS# 1837-57-6 is listed on Canada's NDSL List.
CAS# 1837-57-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1837-57-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
乳酸依沙吖啶概述
乳酸依沙吖啶(Ethacridine lactate),又称利凡诺,是一种吖啶类碱性染料,被认为是染料类中最为有效的防腐剂。未解离成阳离子时,该物质不具备抗菌活性;当其在碱性氮基上带正电荷时,则对革兰阳性菌表现出最强的抑菌作用,并能有效对抗多种化脓菌。乳酸依沙吖啶的抗菌活性与溶液pH值及药物的解离常数相关。通常以0.1%~0.3%水溶液冲洗或使用浸药纱布湿敷来治疗皮肤和黏膜创面感染,在治疗浓度下对组织无损害,但抗菌作用产生较慢,可牢固吸附在黏膜和创面上,作用可持续约一天。此外,其活性会在有机物存在时增强。
理化性质乳酸依沙吖啶是一种黄色结晶性粉末,几乎无臭且味道苦涩。该物质在水中溶解,在热水中易溶,在乙醇中微溶,在乙醚中不溶。其结构式如下图所示:
乳酸依沙吖啶主要用于创面和黏膜的消毒。相关内容由Chemicalbook的侍艳编辑整理(2015-11-17)。
用法用量 注意事项- 溶液在光照下可分解生成剧毒物质。若肉眼观察到本品变为褐绿色,则表明已分解。
- 当溶液中氯化钠浓度高于0.5%时,乳酸依沙吖啶可能从溶液中析出。
- 该溶液遇碱和碘液易产生沉淀。
- 长期使用可能会延缓伤口愈合。
用途:
反应信息
-
作为反应物:描述:参考文献:名称:Moehrle; Von Der Lieck-Waldheim, Scientia Pharmaceutica, 1997, vol. 65, # 1-2, p. 11 - 20摘要:DOI:
-
作为产物:参考文献:名称:CN115850171摘要:公开号:
文献信息
-
Beta-O/S/N fatty acid based compounds as antibacterial and antiprotozoal agents申请人:Ludwig-Maximilians-Universität München公开号:EP2601941A1公开(公告)日:2013-06-12The present invention relates to beta-O/S/N fatty acids and derivatives thereof, in particular the compounds of formula (I) as described and defined herein, and their pharmaceutical use, including their use in the treatment or prevention of bacterial as well as protozoan infections, in particular the treatment or prevention of infections with Gram-positive and/or Gram-negative bacteria and infectious diseases caused by and/or related to Gram-positive and/or Gram-negative bacteria. The invention further relates to the use of these compounds for preventing or eliminating biofilms.
-
[EN] BETA-O/S/N FATTY ACID BASED COMPOUNDS AS ANTIBACTERIAL AND ANTIPROTOZOAL AGENTS<br/>[FR] COMPOSÉS À BASE DE BÊTA-O/S/N ACIDES GRAS EN TANT QU'AGENTS ANTIBACTÉRIENS ET ANTI-PROTOZOAIRES申请人:UNIV MUENCHEN L MAXIMILIANS公开号:WO2013083724A1公开(公告)日:2013-06-13The present invention relates to beta-O/S/N fatty acids and derivatives thereof, in particular the compounds of formula (I) as described and defined herein, and their pharmaceutical use, including their use in the treatment or prevention of bacterial as well as protozoan infections, in particular the treatment or prevention of infections with Gram-positive and/or Gram-negative bacteria and infectious diseases caused by and/or related to Gram-positive and/or Gram-negative bacteria. The invention further relates to the use of these compounds for preventing or eliminating biofilms.
-
Thiirane and michael acceptor compounds and their medical use申请人:Ludwig-Maximilians-Universität München公开号:EP2607361A1公开(公告)日:2013-06-26The present invention relates to thiirane or Michael acceptor compounds, including the compounds of formula (I) as described and defined herein, and pharmaceutical compositions comprising these compounds, as well as their medical use, particularly their use in the treatment or prevention of a bacterial infection, including, e.g., an infection with multidrug-resistant Staphylococcus aureus.
-
GAMMA-AAPEPTIDES WITH POTENT AND BROAD-SPECTRUM ANTIMICROBIAL ACTIVITY申请人:Cai Jianfeng公开号:US20150274782A1公开(公告)日:2015-10-01The present invention is directed to a novel class of antimicrobial agents called γ-AApeptides. The current invention provides various categories of γ-AApeptides, for example, linear γ-AApeptides, cyclic γ-AApeptides, and lipidated γ-AApeptides. γ-AApeptides of the current invention are designed to exert antimicrobial activity while being stable and non-toxic. γ-AApeptides also do not appear to lead to the development of microbial resistance in treated microorganisms. Thus, the disclosed γ-AApeptides can be used for the treatment of various medical conditions associated with pathogenic microorganisms.
-
METHOD FOR PREPARING HIGH ABSORBENT HYDROCOLLOID申请人:LEE Soo-Chang公开号:US20070281029A1公开(公告)日:2007-12-06The present invention relates to a method for preparing a hydrocolloid which may be applied to skin, particularly, wounds. The present invention comprises a first step of preparing a pre-polymer having a viscosity of 200 cps˜10,000 cps by mixing an acryl monomer and an ultraviolet initiator and irradiating on the mixture with ultraviolet rays; a second step of preparing a complex by mixing at least an ultraviolet initiator and a high water absorbing substance with the resulting pre-polymer; and a third step of polymerizing the complex with irradiating ultraviolet rays. The thus prepared hydrocolloid of the present invention has an excellent absorbency as well as self-adhesiveness, is not remained as any residue upon removing it from the skin (wound) and has less skin irritation, without employing any tackifier.
表征谱图
-
氢谱1HNMR
-
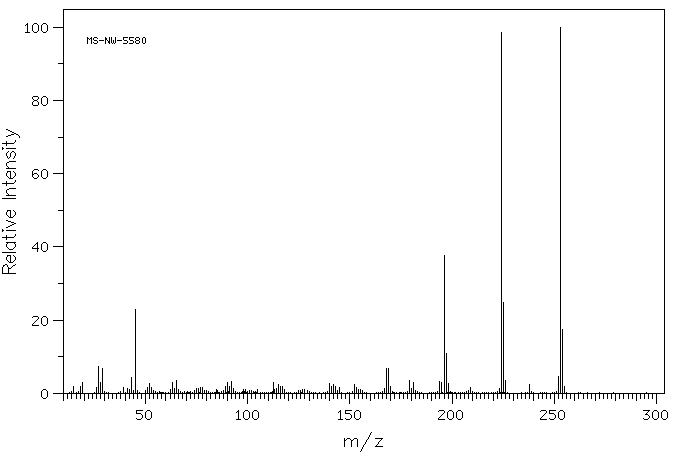
质谱MS
-
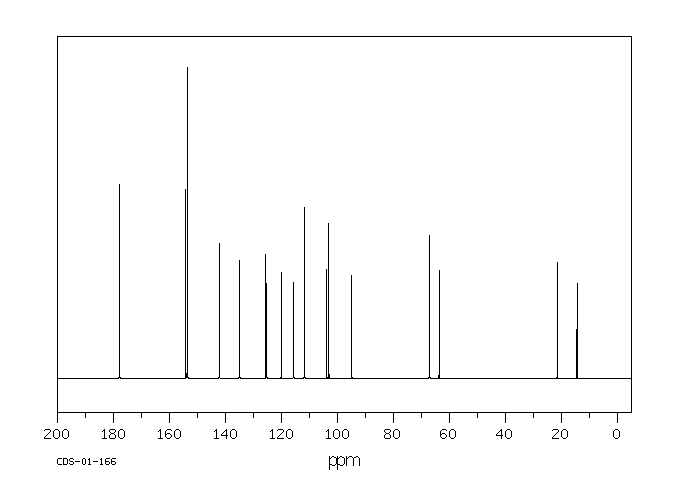
碳谱13CNMR
-
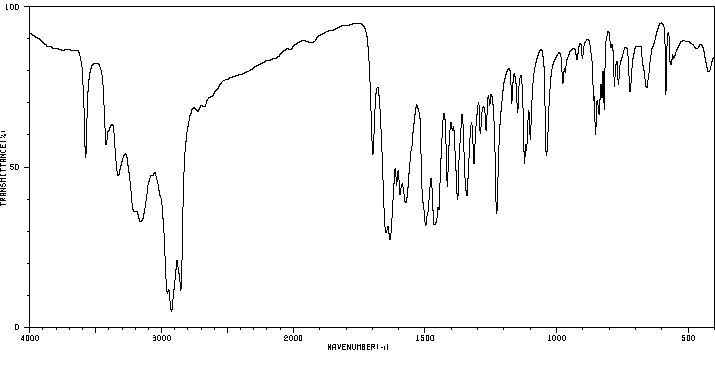
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43