五氟苯甲醇 | 440-60-8
中文名称
五氟苯甲醇
中文别名
2,3,4,5,6-五氟苯甲醇;2,3,4,5,6-五氟苄醇;;5-氟苄醇;2,3',4,4',6-五氯联苯;五氟苄醇;2,3,4,5,6-五氟苄醇
英文名称
(2,3,4,5,6-pentafluorophenyl)methanol
英文别名
pentafluorobenzyl alcohol;2,3,4,5,6-pentafluorobenzyl alcohol;(perfluorophenyl)methanol;(pentafluorophenyl)methanol
CAS
440-60-8
化学式
C7H3F5O
mdl
MFCD00004602
分子量
198.092
InChiKey
PGJYYCIOYBZTPU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:37-38 °C(lit.)
-
沸点:114-115 °C60 mm Hg(lit.)
-
密度:1.4485 (estimate)
-
闪点:190 °F
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:13
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:6
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xn
-
安全说明:S22,S26,S36,S36/37/39
-
危险类别码:R22,R36/37/38
-
WGK Germany:3
-
海关编码:29062900
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:DP0695000
-
危险标志:GHS07
-
危险性描述:H302
-
危险性防范说明:P280,P305+P351+P338
-
储存条件:存放于室温环境,密封保存,并确保干燥。
SDS
模块 1. 化学品
1.1 产品标识符
: 2,3,4,5,6-五氟苯甲醇
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
事故响应
P301 + P312 如果吞咽并觉不适: 立即呼叫解毒中心或就医。
P330 漱口。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H3F5O
分子式
: 198.09 g/mol
分子量
组分 浓度或浓度范围
2,3,4,5,6-pentafluorobenzylic alcohol
<=100%
化学文摘登记号(CAS 440-60-8
No.) 207-126-7
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 37 - 38 °C - lit.
f) 沸点、初沸点和沸程
114 - 115 °C 在 80 hPa - lit.
g) 闪点
88 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 900 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: DP0695000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
化学性质
本品是一种无色固体,熔点为37~38℃,沸点为114~115℃/8kPa,不溶于水,能溶解于苯、醇等有机溶剂。
用途
2,3,4,5,6-五氟苄醇是卫生用杀虫剂五氟苯菊酯的中间体,同时在医药、农药和液晶材料领域也作为重要中间体使用。
生产方法
该产品的制备方法为:将2,3,4,5,6-五氟苯甲酸在锌电极作用下于5%硫酸溶液中进行流动床电解,从而得到所需产品。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,3,4,5,6-五氟甲苯 2,3,4,5,6-pentafluorotoluene 771-56-2 C7H3F5 182.093 2,3,4,5,6-五氟苯甲酸 Pentafluorobenzoic acid 602-94-8 C7HF5O2 212.076 五氟苯甲酸甲酯 methyl pentafluorobenzoate 36629-42-2 C8H3F5O2 226.103 五氟苯甲醛 perfluorobenzaldehyde 653-37-2 C7HF5O 196.076 2,3,4,5,6-五氟苄基氯 2,3,4,5,6-pentafluorobenzyl chloride 653-35-0 C7H2ClF5 216.538 α-溴-2,3,4,5,6-五氟甲基苯酸酯 (bromomethyl)pentafluorobenzene 1765-40-8 C7H2BrF5 260.989 (五氟苯基)甲胺 2,3,4,5,6-pentafluorobenzylamine 1548-77-2 C7H4F5N 197.107 2,3,4,5,6-五氟苯甲酸酐 pentafluorobenzoic anhydride 15989-99-8 C14F10O3 406.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3,4,5-四氟苯甲醇 2,3,4,5-tetrafluorobenzyl alcohol 53072-18-7 C7H4F4O 180.102 2,3,5,6-四氟苯甲醇 2,3,5,6-tetrafluorobenzyl alcohol 4084-38-2 C7H4F4O 180.102 —— 1-(methoxymethyl)-2,3,4,5,6-pentafluorobenzene 66286-23-5 C8H5F5O 212.119 —— bis-perfluorobenzyl ether —— C14H4F10O 378.169 —— Methanoic acid pentafluorobenzyl ester —— C8H3F5O2 226.103 —— 2-methyl-3,4,5,6-tetrafluorobenzyl alcohol —— C8H6F4O 194.129 2,3,4,5,6-五氟甲苯 2,3,4,5,6-pentafluorotoluene 771-56-2 C7H3F5 182.093 —— pentafluorophenylmethoxytrimethylsilane 1189949-29-8 C10H11F5OSi 270.274 —— Ethanoic acid pentafluorobenzyl ester 2002-93-9 C9H5F5O2 240.13 2,3,4,5,6-五氟氯甲酸苄酯 pentafluorobenzyl chlorocarbonate 53526-74-2 C8H2ClF5O2 260.548 2,3,4,5,6-五氟苯甲酸 Pentafluorobenzoic acid 602-94-8 C7HF5O2 212.076 2,3,4,5-四氟苯甲酸 2,3,4,5-tetrafluorobenzoic acid 1201-31-6 C7H2F4O2 194.085 —— 2,3,4,5,6-pentafluoro-2'-<(methylsulfonyl)oxy>toluene 111196-49-7 C8H5F5O3S 276.184 —— 2,3,4,5,6-pentafluorobenzyl acrylate 153614-61-0 C10H5F5O2 252.141 —— Propanoic acid pentafluorobenzyl ester 21634-97-9 C10H7F5O2 254.156 —— (pentafluorophenyl)ylmethyl sulfamate 1306748-10-6 C7H4F5NO3S 277.172 (2,3,4,5,6-五氟苯基)甲基丁酸酯 Butanoic acid pentafluorobenzyl ester 21634-99-1 C11H9F5O2 268.183 —— 2-Methylpropanoic acid pentafluorobenzyl ester 21634-98-0 C11H9F5O2 268.183 —— benzoic acid pentafluorobenzyl ester 21635-04-1 C14H7F5O2 302.2 —— Pentanoic acid pentafluorobenzyl ester 21635-00-7 C12H11F5O2 282.21 五氟苯甲醛 perfluorobenzaldehyde 653-37-2 C7HF5O 196.076 —— Pentafluorobenzyl hexanoate 21635-01-8 C13H13F5O2 296.237 —— Succinic acid pentafluorobenzyl ester —— C18H8F10O4 478.243 (2,3,4,5,6-五氟苯基)甲基十二烷酸酯 Dodecanoic acid pentafluorobenzyl ester 21635-07-4 C19H25F5O2 380.398 —— Octanoic acid pentafluorobenzyl ester 21635-03-0 C15H17F5O2 324.291 2,3,4,5,6-五氟苄基氯 2,3,4,5,6-pentafluorobenzyl chloride 653-35-0 C7H2ClF5 216.538 α-溴-2,3,4,5,6-五氟甲基苯酸酯 (bromomethyl)pentafluorobenzene 1765-40-8 C7H2BrF5 260.989 —— 2,3,5,6-tetrafluoro-4-(hydroxymethyl)benzoic acid 107900-84-5 C8H4F4O3 224.112 —— 2,3,4,5,6-pentafluorobenzyl fluoride 22006-43-5 C7H2F6 200.083 —— 4-amino-2,3,5,6-tetrafluorobenzyl alcohol 17078-34-1 C7H5F4NO 195.116 —— pentafluorobenzyl 3-phenylpropanoate 62434-95-1 C16H11F5O2 330.254 —— 2,3,4,5,6-pentafluorobenzyl 2-methyl-3-oxobutanoate 1003014-19-4 C12H9F5O3 296.194 2,3,4,5,6-五氟苯乙胺 2-(2,3,4,5,6-pentafluorophenyl)ethan-1-amine 1583-76-2 C8H6F5N 211.134 5-氟苯乙睛 (pentafluorophenyl)acetonitrile 653-30-5 C8H2F5N 207.103 2-(五氟苯基)乙醇 2-(pentafluorophenyl)ethanol 653-31-6 C8H5F5O 212.119 —— 1-benzyl-2,3,4,5,6-pentafluorobenzene 7484-19-7 C13H7F5 258.191 —— 1,2,3,4,5-pentafluoro-6-(4-fluorobenzyl)benzene 1234340-41-0 C13H6F6 276.181 —— O-(2,3,4,5,6-pentafluorobenzyl)-N,N'-diisopropylisourea —— C14H17F5N2O 324.294 4-甲氧基-2,3,5,6-四氟苯甲醇 (2,3,5,6-tetrafluoro-4-methoxyphenyl)methanol 35175-79-2 C8H6F4O2 210.128 —— 4-methylthio-2,3,5,6-tetrafluorobenzyl alcohol 106666-01-7 C8H6F4OS 226.195 —— 4-hydrazino-2,3,5,6-tetrafluorobenzyl alcohol 121038-08-2 C7H6F4N2O 210.131 1,2,3,4,5-五氟-6-[2-(2,3,4,5,6-五氟苯基)乙基]苯 2,2',3,3',4,4',5,5',6,6'-decafluorobibenzyl 853-74-7 C14H4F10 362.17 2-(2,3,4,5,6-五氟苯基)乙酰胺 2.3.4.5.6-Pentafluor-phenylacetamid 653-20-3 C8H4F5NO 225.118 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:参考文献:名称:应变和稳定性的经验教训:(+)-[5]-梯烷酸的对映选择性合成。摘要:结构复杂且高度紧张的天然产物的合成为新反应和策略的开发提供了独特的挑战和意想不到的机会。在此,报道了(+)-[5]-梯烷酸的合成。在到达目标的途中,发现了不寻常和意想不到的应变释放驱动的转化。这一事件需要对合成设计进行彻底修改,最终导致通过烯丙基硼化/Zweifel 烯化序列开发出新型逐步环丁烷组装。DOI:10.1002/anie.201910901
-
作为产物:描述:参考文献:名称:原位硅烷活化能够催化还原羧酸摘要:我们描述了使用亚化学计量乙酸锌和N-甲基吗啉与苯基硅烷作为标称末端还原剂结合使用将羧酸转化为醇的催化系统。通过19 F NMR 光谱监测反应表明,反应通过原位生成甲硅烷基酯中间体使羧酸和硅烷相互活化而进行。DOI:10.1039/d1cc03396d
-
作为试剂:描述:C16H17BrO3 在 甲醇 、 bis-triphenylphosphine-palladium(II) chloride 、 sodium tetrahydroborate 、 三甲基氯硅烷 、 三氟甲磺酸三甲基硅酯 、 碘苯二乙酸 、 五氟苯甲醇 、 水 、 C40H39ClN4OPRu(1+)*Cl(1-) 、 对甲苯磺酸 、 三乙胺 、 copper(I) bromide 作用下, 以 四氢呋喃 、 正己烷 、 二氯甲烷 、 N,N-二甲基甲酰胺 、 甲苯 、 1,1,2,2-四氯乙烷 为溶剂, 反应 49.5h, 生成 cephanolide C参考文献:名称:Cephalotane 型二萜类 Cephanolides A-D 的不对称全合成摘要:Cephanolides A-D 是头孢烷型二萜类化合物,具有嵌入桥接 δ-内酯的新型 6/6/6/5 四环核心。头孢内酯 A-D 的不对称和发散的全合成已经完成,从已知的醇开始进行 11-14 步。本工作的突出特点包括(i)底物控制的非对映选择性分子间 Diels-Alder 反应形成 6-6顺式稠环,(ii)钯催化的形式双分子 [2 + 2 + 2] 环加成反应通过涉及多个碳金属化的部分分子间级联反应序列快速安装关键的四环骨架,以及(iii)内酯化和后期氧化多样化以完成四种苯类头孢内酯的全合成。DOI:10.1021/jacs.2c03978
文献信息
-
Copper bis(oxazolines) as catalysts for stereoselective aziridination of styrenes with N-tosyloxycarbamates作者:Hélène Lebel、Michaël Parmentier、Olivier Leogane、Karen Ross、Cédric SpitzDOI:10.1016/j.tet.2012.02.044日期:2012.4styrenes. Subsequent ring-opening reactions of styrene-derived aziridines at the benzylic position were observed with various oxygen and nitrogen nucleophiles under Lewis acid catalysis affording the corresponding products with complete inversion of stereochemistry. The strategy was used to synthesize the β-blocker, (R)-nifenalol.
-
Substituted N, N-disubstituted diamino compounds useful for inhibiting cholesteryl ester transfer protein activity申请人:——公开号:US20020120011A1公开(公告)日:2002-08-29The invention relates to substituted polycyclic aryl and heteroaryl tertiary-heteroalkylamine compounds useful as inhibitors of cholesteryl ester transfer protein (CETP; plasma lipid transfer protein-I) and compounds, compositions and methods for treating atherosclerosis and other coronary artery diseases. Preferred tertiary-heteroalkylamine compounds are substituted N,N-disubstituted diamines. A preferred specific N,N-disubstituted diamine is the compound: 1
-
Protection of hydroxy groups as trimethylsilyl ethers using 1,1,1,3,3,3-hexamethyldisilazane (HMDS) catalyzed by poly(4-vinylpyridinium tribromide)作者:Arash Ghorbani-Choghamarani、Mohammad Ali Zolfigol、Maryam Hajjami、Khorshid Darvishi、Laleh GholamniaDOI:10.1135/cccc2009560日期:——
A very efficient procedure for the protection of alcohols and phenols is presented. The mixture of 1,1,1,3,3,3-hexamethyldisilazane (HMDS) and catalytic amounts of poly(4-vinylpyridinium tribromide) was found to be effective for the trimethylsilylation of alcohols and phenols. Protection reaction is very simple and performs heterogeneously in acetonitrile at room temperature under mild conditions.
-
Deoxytrifluoromethylthiolation and Selenylation of Alcohols by Using Benzothiazolium Reagents作者:Stefan Dix、Michael Jakob、Matthew N. HopkinsonDOI:10.1002/chem.201901607日期:2019.6.7Aliphatic compounds substituted with medicinally important trifluoromethylthio (SCF3) and trifluoromethylselenyl (SeCF3) groups were synthesized directly from alcohols by using the new benzothiazolium salts BT‐SCF3 and BT‐SeCF3. These bench‐stable fluorine‐containing reagents are facile to use and can be prepared in two steps from non‐fluorinated heteroaromatic starting materials. The metal‐free d
-
Synthesis, in Vitro Pharmacology, Structure−Activity Relationships, and Pharmacokinetics of 3-Alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic Acid Derivatives as Potent and Selective Group II Metabotropic Glutamate Receptor Antagonists作者:Atsuro Nakazato、Kazunari Sakagami、Akito Yasuhara、Hiroshi Ohta、Ryoko Yoshikawa、Manabu Itoh、Masato Nakamura、Shigeyuki ChakiDOI:10.1021/jm0400294日期:2004.8.1Novel group II metabotropic glutamate receptor (mGluR) antagonists, 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives 11 and 12, were discovered by the incorporation of a hydroxy or alkoxyl group onto the C-3 portion of selective and potent group II mGluR agonist 5, (1R,2S,5R,6R)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid. Among these compounds, (1R,2R通过掺入羟基或烷氧基,发现了新型的II类代谢型谷氨酸受体(mGluR)拮抗剂3-烷氧基-2-氨基-6-氟双环[3.1.0]己烷-2,6-二羧酸衍生物11和12。在选择性和有效的第II组mGluR激动剂5(1R,2S,5R,6R)-2-氨基-6-氟双环[3.1.0]己烷-2,6-二羧酸的C-3部分上连接基团 在这些化合物中,(1R,2R,3R,5R,6R)-2-氨基-3-(3,4-二氯苄氧基)-6-氟双环[3.1.0]己烷-2,6-二羧酸(-)- 11be(MGS0039)是一种具有最佳药代动力学特征的高选择性强效II组mGluR拮抗剂。化合物(-)-11对mGlu 2(Ki = 2.38 +/- 0.40 nM)和mGlu 3(4.46 +/- 0.31 nM)表现出高亲和力,但对mGluR 7(Ki = 664 +/- 106 nM)的亲和力低,和有效的mGlu 2拮抗剂活性(IC50 =
表征谱图
-
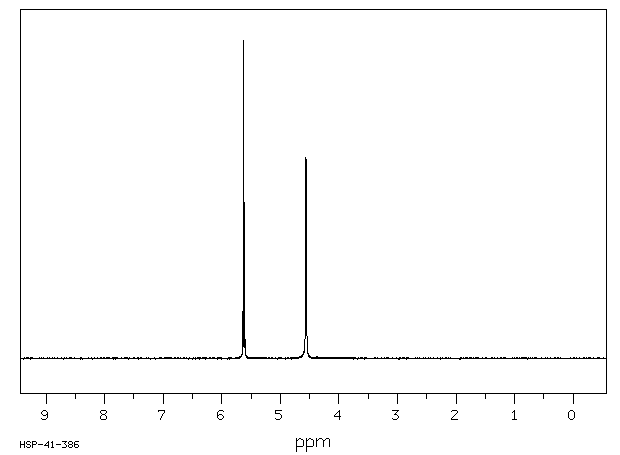
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
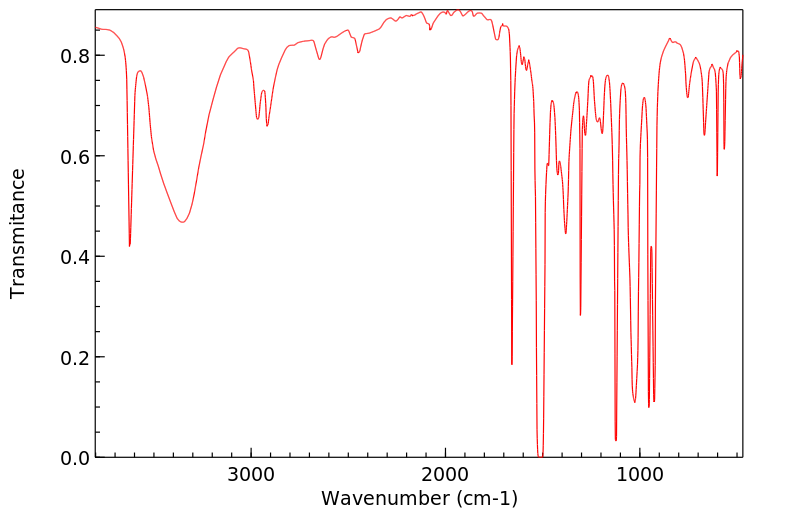
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫