仲丁醚 | 6863-58-7
物质功能分类
中文名称
仲丁醚
中文别名
仲丁基醚;双(2-丁基)醚
英文名称
di-2-butyl ether
英文别名
Di-sec-butyl ether;2-butan-2-yloxybutane
CAS
6863-58-7
化学式
C8H18O
mdl
MFCD00039921
分子量
130.23
InChiKey
HHBZZTKMMLDNDN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-100°C
-
沸点:121 °C (lit.)
-
密度:0.759 g/mL at 25 °C (lit.)
-
闪点:78 °F
-
蒸汽压力:16.25 mmHg
-
保留指数:786
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:9
-
可旋转键数:4
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3
-
危险品标志:Xn
-
危险类别码:R20,R10,R
-
WGK Germany:3
-
RTECS号:EQ2220000
-
海关编码:2909199090
-
危险品运输编号:UN 1149 3/PG 3
-
储存条件:库房应保持通风、低温和干燥的环境,并将储存物品与氧化剂及食品原料分开存放。
SDS
sec-Butyl Ether (DL- and meso- mixture) (stabilized with Revision number: 6
HQ)
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: sec-Butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Flammable liquid and vapour
Hazard statements
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Other hazards which do not May cause polimerization.
result in classification
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
sec-Butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Components:
Percent: >97.0%(GC)
6863-58-7
CAS Number:
Synonyms: Di-sec-butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Chemical Formula: C8H18O
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising This substance may polimerize explosively when heated or involved in a fire.
from the chemical: Container may explode when heated. Combat fire from a sheltered position.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into a
containment and cleaning covered container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Almost colorless
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 121°C
Flash point: 26°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.76
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Polymerization may occur under the influences of heat, light or on contact with
polymelization initiators such as peroxides etc.
Possibility of hazardous No special reactivity has been reported.
reactions:
Heat, Spark, Open flame, Static discharge, Light
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
ihl-mus LC50:130 g/m3/15M
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: EQ2220000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 1149
Proper shipping name: Dibutyl ethers
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
HQ)
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: sec-Butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Revision number: 6
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS
Category 3
Flammable liquids
HEALTH HAZARDS Not classified
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Flammable liquid and vapour
Hazard statements
Precautionary statements:
Keep away from heat/sparks/open flames/hot surfaces. - No smoking.
[Prevention]
Keep container tightly closed.
Use explosion-proof electrical/ventilating/lighting equipment. Take precautionary
measures against ignition by the static discharge and the spark.
Wear protective gloves/eye protection/face protection.
[Response] IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
[Storage] Store in a well-ventilated place. Keep cool.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Other hazards which do not May cause polimerization.
result in classification
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
sec-Butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Components:
Percent: >97.0%(GC)
6863-58-7
CAS Number:
Synonyms: Di-sec-butyl Ether (DL- and meso- mixture) (stabilized with HQ)
Chemical Formula: C8H18O
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, carbon dioxide.
media:
Unsuitable extinguishing Water (It may scatter and spread fire.)
media:
Specific hazards arising This substance may polimerize explosively when heated or involved in a fire.
from the chemical: Container may explode when heated. Combat fire from a sheltered position.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Keep containers cool by
spraying with water. Eliminate all ignition sources if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in dry sand or inert absorbent before recovering it into a
containment and cleaning covered container. In case of large amount of spillage, contain a spill by bunding.
up: Adhered or collected material should be promptly disposed of, in accordance with
appropriate laws and regulations.
Prevention of secondary Remove all sources of ignition. Fire-extinguishing devices should be prepared in
hazards: case of a fire. Use spark-proof tools and explosion-proof equipment.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Keep away from heat/sparks/open flame/hot
surfaces. -No smoking. Take measures to prevent the build up of electrostatic
charge. Use explosion-proof equipment. Wash hands and face thoroughly after
handling.
Use a closed system if possible. Use a ventilation, local exhaust if vapour or aerosol
will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool, dark and well-ventilated place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
Clear
Form:
Colour: Colorless - Almost colorless
No data available
Odour:
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 121°C
Flash point: 26°C
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: 0.76
Solubility(ies):
[Water] No data available
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Polymerization may occur under the influences of heat, light or on contact with
polymelization initiators such as peroxides etc.
Possibility of hazardous No special reactivity has been reported.
reactions:
Heat, Spark, Open flame, Static discharge, Light
Conditions to avoid:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
ihl-mus LC50:130 g/m3/15M
Acute Toxicity:
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
No data available
IARC =
NTP = No data available
Reproductive toxicity: No data available
RTECS Number: EQ2220000
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
sec-Butyl Ether (DL- and meso- mixture)
(stabilized with HQ)
Section 12. ECOLOGICAL INFORMATION
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 3: Flammable liquid.
UN-No: 1149
Proper shipping name: Dibutyl ethers
Packing group: III
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
类别:易燃液体
毒性分级:高毒
急性毒性:吸入-小鼠 LC50: 130 毫克/立方米(15 分钟)
可燃性危险特性:遇热、明火及氧化剂易燃;受热分解后排出有毒且具有辛辣刺激性的烟雾
储运特性:应存放在库房通风、低温和干燥的环境中,并与氧化剂和食品原料分开存放
灭火剂:二氧化碳、干粉或泡沫
反应信息
-
作为反应物:参考文献:名称:Kessel, Justus Liebigs Annalen der Chemie, 1875, vol. 175, p. 50摘要:DOI:
-
作为产物:参考文献:名称:丁醇异构体对纤维素对液体和固体酸催化剂催化的乙酰丙酸丁酯合成反应性的影响†摘要:乙酰丙酸的丁酯形成一类有趣的生物基化合物,可用作例如燃料添加剂。它们的制备主要通过乙酰丙酸的酯化进行,而很少有报道报道从纤维素直接合成乙酰丙酸的信息有限。在目前的工作中,我们首次详细研究了丁醇异构体对非催化纤维素液化和酸催化纤维素中乙酰丙酸丁酯形成的影响。在没有催化剂的情况下,醇类对液化没有影响,在300°C下放置2小时后,其达到70-85%。在催化剂的存在下,我们表明醇的种类对乙酰丙酸丁酯的产率有显着影响。在存在H的情况下,伯醇的收率为50%2 SO 4(200°C,30分钟)。对于涉及纤维素的这类一锅转化,这种产率水平可以认为是非常有趣的。使用仲醇时,收率低于20%,而叔醇则不会形成乙酰丙酸丁酯。我们还首次报道了在固体酸存在下的这种转变。尽管失活,不溶的Cs 2 HPW 12 O 40或硫酸化的氧化锆仍能不均一地催化反应,导致收率有限(13%)(200°C,1小时)。我们最终表明,丁醇DOI:10.1039/c5nj02493e
文献信息
-
[EN] PROCESS FOR PREPARING ACYLPHOSPHANES AND DERIVATIVES THEREOF<br/>[FR] PROCEDE DE PREPARATION D'ACYLPHOSPHANES ET DE DERIVES D'ACYLPHOSPHANES申请人:CIBA SC HOLDING AG公开号:WO2005014605A1公开(公告)日:2005-02-17The present invention relates to a new, selective process for the preparation of mono- and bisacylphosphanes of formula (I) n and m are each independently of the other 1 or 2; R1, if n = 1, is e.g. phenyl R1, if n = 2, is e.g. C1-C18alkylene or phenylene; R2 is e. g. C1-C18alkyl, phenyl or substituted phenyl; R3 is e. g. C1-C18alkyl, by (1) reacting a phosphorous halide of formula Ila or a phosphorous halide oxide of formula (Ilb) or a phosphorous halide sulfide of formula (IIc) with an alkali metal in a solvent in the presence of a proton source; (2) subsequent reaction with m acid halides of formula (III) An oxidation step may follow to obtain mono- and bisacylphosphane oxides or mono-and bisacylphosphane sulfides.
-
Chemical conversions using sheet silicates: novel intermolecular dehydrations of alcohols to ethers and polymers作者:James A. Ballantine、Mary Davies、Howard Purnell、Mongkon Rayanakorn、John M. Thomas、Kevin J. WilliamsDOI:10.1039/c39810000427日期:——Aliphatic primary alcohols, when intercalated in certain ion-exchanged montmorillonites, react preferentially via an intermolecular nucleophilic displacement of water to give high yields of di-(alk-1-yl) ethers, rather than the competitive intramolecular dehydration to alkenes; an essentially similar process yields polymeric material, poly(phenylenemethylene), from benzyl alcohol, but aliphatic secondary
-
Catalyst composition for hydrogenation and method for hydrogenation using the same申请人:Asahi Kasei Chemicals Corporation公开号:US10016749B2公开(公告)日:2018-07-10A catalyst composition for hydrogenation including (A) to (D), in which a mass ratio ((C)/(A)) is 0.1 to 4.0 and a mass ratio ((D)/(A)) is 0.01 to 1.00, (A): a titanocene compound represented by formula (1), (wherein R5 and R6 are any group selected from hydrogen, a hydrocarbon group having 1 to 12 carbon atoms, an aryloxy group, an alkoxy group, a halogen group, and a carbonyl group. R1 and R2 are any group selected from the group consisting of hydrogen and a hydrocarbon group having 1 to 12 carbon atoms, and R1 and R2 are not all hydrogen atoms or all a hydrocarbon group having 1 to 12 carbon atoms), (B): a reductant formed from a compound containing an element selected from the elements Li, Na, K, Mg, Zn, Al, and Ca, (C): an unsaturated compound having a molecular weight of 400 or less, and (D): a polar compound.
-
[EN] ISOINDOLINE DERIVATIVES COMPRISING PHENYL GROUPS AND THEIR USE IN THE TREATMENT OF PAIN DISORDERS<br/>[FR] DÉRIVÉS ISOINDOLINE COMPRENANT DES GROUPES PHÉNYLES ET LEUR UTILISATION DANS LE TRAITEMENT DE TROUBLES DE LA DOULEUR申请人:ASTRAZENECA AB公开号:WO2009145721A1公开(公告)日:2009-12-03Compounds of formula I are claimed, (I) wherein R1 is hydrogen, C1-3alkyl, Ci_3alkoxy, cyano, hydroxy or halo; and wherein said Ci^alkyl is optionally substituted by one or more substituents independently selected from hydroxy, Ci^alkoxy andfluoro; and said Ci^alkoxy is optionally substituted by one or morefluoro; m is 1 or 2; R2 and R3 is each and independently selected from hydrogen, Ci_4haloalkyl, Ci_4haloalkoxy, halo, Ci_4alkoxy, Ci_4alkyl and C3_7cycloalkyloxy; wherein said C3. γcycloalkyloxy is optionally substituted by one or morefluoro; and R2 and R3 may not both be hydrogen; D is C3_7cycloalkyl or C3_7heterocycloalkyl; and wherein said Cs-jcycloalkyl or Cs- γheterocycloalkyl may optionally be substituted by one or more X*; X4 is halo, Ci_3alkyl, Ci_3alkyl0Ci_3alkyl, Ci_3alkoxy, benzyl, Ci_4alkylsulfonyl, oxo, R4O(C=O), R5(C=O), or C5.6 heteroaryl; wherein said Cisalkyl, CisalkylOCisalkyl, Ci^alkoxy and C i^alkylsulfonyl is optionally substituted by one or more fluoro; R4 is Ci_4alkyl, Ci_4alkyl0Ci_4alkyl, C5_6cycloalkyl, or aryl; R5 is Ci_4alkyl, Ci_4fluoroalkyl or Cs_6 heteroaryl; Li is Ci_4alkylene or a bond; L2 is Ci_3alkylene; with the exception of the compound 2-(cyclohexylmethyl)-3-oxo-N-[2-(trifluoromethyl)benzyl]isoindoline-l-carboxamide; as well as a pharmaceutically acceptable salt, or isomer thereof, or a salt of said isomer. The compounds of the invention are useful in therapy such as pain therapy.公式I的化合物被要求,其中R1是氢,C1-3烷基,Ci_3烷氧基,氰基,羟基或卤素基;其中所述Ci^烷基可以选择性地被一个或多个取代基独立地选择自羟基,Ci^烷氧基和氟基;所述Ci^烷氧基可以选择性地被一个或多个氟基取代;m为1或2;R2和R3分别且独立地选择自氢,Ci_4卤代烷基,Ci_4卤代烷氧基,卤素,Ci_4烷氧基,Ci_4烷基和C3_7环烷氧基;其中所述C3. γ环烷氧基可以选择性地被一个或多个氟基取代;且R2和R3不能同时为氢;D为C3_7环烷基或C3_7杂环烷基;其中所述Cs-j环烷基或Cs-γ杂环烷基可以选择性地被一个或多个X*取代;X4为卤素,Ci_3烷基,Ci_3烷基0Ci_3烷基,Ci_3烷氧基,苄基,Ci_4烷基磺酰基,氧代,R4O(C=O),R5(C=O),或C5.6杂环烷基;其中所述Ci烷基,Ci烷基0Ci烷基,Ci^烷氧基和Ci^烷基磺酰基可以选择性地被一个或多个氟基取代;R4为Ci_4烷基,Ci_4烷基0Ci_4烷基,C5_6环烷基,或芳基;R5为Ci_4烷基,Ci_4氟烷基或Cs_6杂环烷基;Li为Ci_4烷基或键;L2为Ci_3烷基;除了化合物2-(环己基甲基)-3-氧代-N-[2-(三氟甲基)苄基]异吲哚啉-1-羧酰胺;以及其药学上可接受的盐,或其异构体的盐。本发明的化合物在治疗中如疼痛治疗中是有用的。
-
[EN] ISOINDOLINE DERIVATIVES COMPRISING AN ADDITIONAL HETEROCYCLIC GROUP AND THEIR USE IN THE TREATMENT OF PAIN DISORDERS<br/>[FR] DÉRIVÉS ISOINDOLINE COMPRENANT UN GROUPE HÉTÉROCYCLIQUE SUPPLÉMENTAIRE ET LEUR UTILISATION DANS LE TRAITEMENT DE TROUBLES DE LA DOULEUR申请人:ASTRAZENECA AB公开号:WO2009145718A1公开(公告)日:2009-12-03Compounds of formula I are claimed, wherein R1is hydrogen, C1_3alkyl, C1_3alkoxy, cyano, hydroxy or halo; wherein C1-3alkyl may optionally be substituted by one or more substituents independently selected from hydroxy, C1-3alkoxy orfluoro; and wherein Ci^alkoxy may optionally be substituted by one or more fluoro; m is 1 or 2; R2 and R3 is each and independently selected from hydrogen, Ci_4haloalkyl, C1_4haloalkoxy, halo, C1_4alkoxy, C1_4alkyl and C3_7cycloalkyloxy; and wherein said C3_7cycloalkyloxy may optionally be substituted by one or more fluoro; and whereas both R2 and R3 can not be hydrogen; Het is selected from any one of pyridinyl, pyrazinyl, isoxazolyl, pyrazolyl, indolyl, triazolyl and pyrimidinyl, wherein each such heteroaryl may optionally be substituted by one or more X4; X4 is halo, C1-3alkyl, C1-3alkyl0C1-3alkyl, -CH(CH3)-O-C(CH3)3,C1_4alkoxy, cyano, or hydroxyl, or Ci_2hydroxyalkyl;; and wherein said C1-3alkyl, C 1-3alkylOC1-3alkyl, -CH(CH3)-O-C(CH3)3, or C1_4alkoxy may each optionally be substituted by one or more fluoro; L1 is C1_4alkylene, which may optionally be fluorinated or hydroxylated; and L2 is C1-3alkylene; with the exception of the compounds: 2-[1-(1,5-dimethyl-lH-pyrazol-4-yl)ethyl]-5,7-dimethoxy-3-oxo-N-[2-(trifluoromethyl)benzyl]isoindoline-1-carboxamide; N-(4-fluorobenzyl)-3-oxo-2-(-pyridin-4-yletyl)isoindoline-1-carboxamide and N-(2-chlorobenzyl)-2[2-(1H-indol-3-yl)-1-methyletyl]-3-oxoisoindoline-1-carboxamide; The invention further relates to pharmaceutical compositions containing said compounds and to the use of said compounds in therapy.公式I的化合物被要求,其中R1是氢,C1-3烷基,C1-3烷氧基,氰基,羟基或卤素;其中C1-3烷基可以选择性地被一个或多个取代基取代,这些取代基独立地选自羟基,C1-3烷氧基或氟基;而C1-3烷氧基可以选择性地被一个或多个氟基取代;m为1或2;R2和R3分别且独立地选自氢,C1-4卤代烷基,C1-4卤代烷氧基,卤素,C1-4烷氧基,C1-4烷基和C3-7环烷氧基;其中所述的C3-7环烷氧基可以选择性地被一个或多个氟基取代;而且R2和R3都不能是氢;Het选自吡啶基,吡嗪基,异噁唑基,吡唑基,吲哚基,三唑基和嘧啶基中的任何一种,其中每种这样的杂环烷基可以选择性地被一个或多个X4取代;X4是卤素,C1-3烷基,C1-3烷氧基C1-3烷基,-CH(CH3)-O-C( )3,C1-4烷氧基,氰基,或羟基,或C1-2羟基烷基;而所述的C1-3烷基,C1-3烷氧基C1-3烷基,-CH( )-O-C( )3,或C1-4烷氧基可以选择性地被一个或多个氟基取代;L1是C1-4烷基,可以选择性地被氟化或羟基化;而L2是C1-3烷基;除了以下化合物:2-[1-(1,5-二甲基-1H-吡唑-4-基)乙基]-5,7-二甲氧基-3-氧代-N-[2-(三氟甲基)苯甲基]异吲哚啉-1-羧酰胺;N-(4-氟苯甲基)-3-氧代-2-(-吡啶-4-基乙基)异吲哚啉-1-羧酰胺和N-(2-氯苯甲基)-2[2-(1H-吲哚-3-基)-1-甲基乙基]-3-氧代异吲哚啉-1-羧酰胺;本发明还涉及含有所述化合物的药物组合物以及所述化合物在治疗中的使用。
表征谱图
-
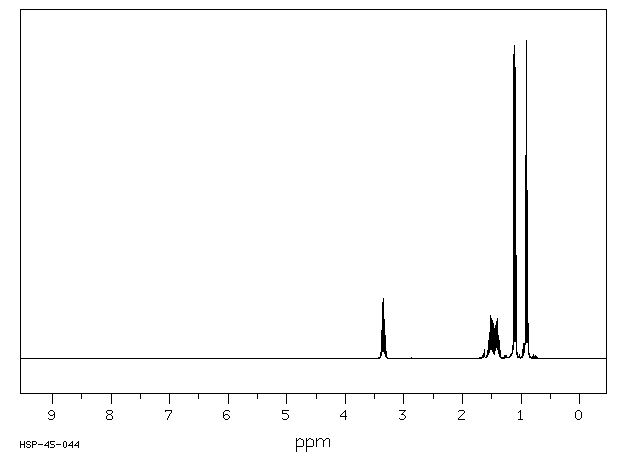
氢谱1HNMR
-
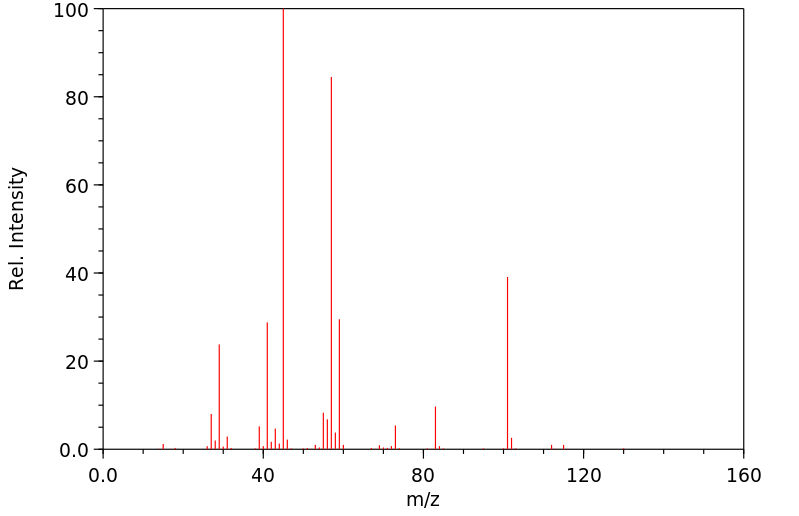
质谱MS
-
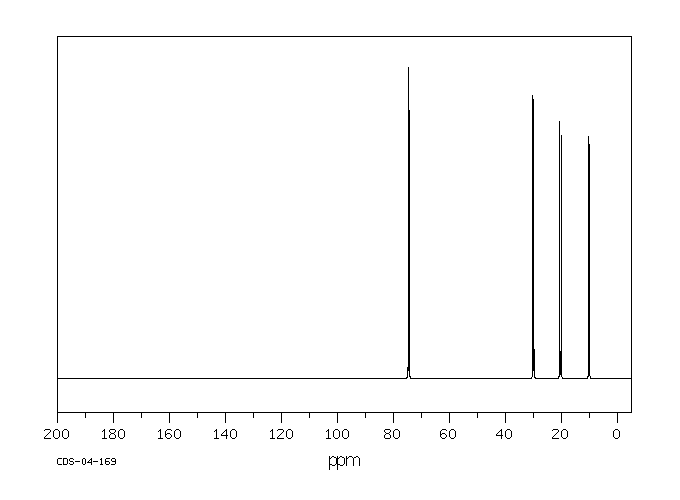
碳谱13CNMR
-
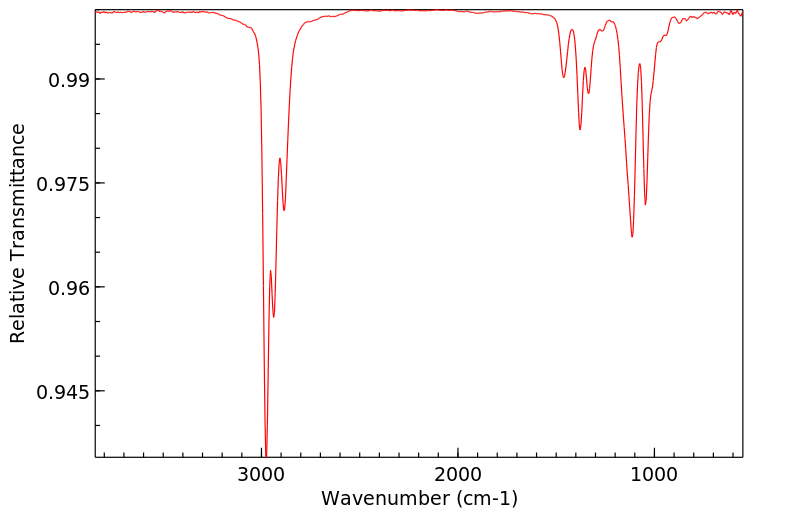
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷