倍他米松 | 378-44-9
物质功能分类
分子结构分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:235-237°C
-
比旋光度:D +108° (acetone)
-
沸点:568.2±50.0 °C(Predicted)
-
密度:1.1283 (estimate)
-
溶解度:几乎不溶于水,微溶于无水乙醇,极微溶于二氯甲烷。
-
LogP:2.01 at 25℃
-
物理描述:Solid
-
颜色/状态:Crystals from ethyl acetate
-
气味:ODORLESS
-
水溶性:-3.77
-
稳定性/保质期:
HYGROSCOPIC. /SODIUM PHOSPHATE/
-
旋光度:Specific optical rotation: +63.5 deg @ 24 °C/D (dioxane)
-
碰撞截面:186.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:28
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.73
-
拓扑面积:94.8
-
氢给体数:3
-
氢受体数:6
ADMET
安全信息
-
危险品标志:Xn,Xi
-
安全说明:S22,S36
-
危险类别码:R40
-
WGK Germany:2
-
海关编码:2937229000
-
危险品运输编号:HAZARD
-
RTECS号:TU4000000
SDS
制备方法与用途
倍他米松介绍
倍他米松又称培他美松、贝皮质醇、贝氟美松、培氟美松,属于肾上腺皮质激素类药物。它是地塞米松的同分异构体,作用与泼尼松龙和地塞米松相同。该药物具有抗炎、抗风湿、抗过敏和抑制免疫等多种药理作用。
倍他米松的抗炎作用显著强于其他几种常见肾上腺皮质激素(如地塞米松、曲安西龙、氢化可的松)。它可以减轻和防止组织对炎症的反应,减少局部非感染性炎症引起的发热、发红及肿胀。根据研究,0.3毫克倍他米松的抗炎效果与地塞米松0.75毫克、泼尼松5毫克或可的松25毫克相当。
临床应用
倍他米松用于治疗多种疾病和症状,包括活动性风湿病、类风湿性关节炎、红斑狼疮、严重支气管哮喘、严重皮炎、急性白血病、过敏性皮炎、湿疹、神经性皮炎、脂溢性皮炎及瘙痒症等。此外,在某些感染的综合治疗中也有应用。
禁忌症
倍他米松禁用于有严重精神病史、活动性十二指肠或胃溃疡、新近进行过胃肠吻合手术、较重骨质疏松、明显糖尿病、严重高血压,以及病毒、细菌、霉菌感染未能控制的情况下(如脓疱病、体癣、股癣等)。
不良反应
长期使用倍他米松常见的不良反应包括医源性库欣综合征面容和体态改变、体重增加、下肢浮肿、紫纹、易出血倾向、创口愈合不良、痤疮、月经紊乱、骨质疏松及骨折(如脊椎压缩性骨折、长骨病理性骨折)、肌无力、肌萎缩、低血钾综合征、胃肠道刺激(恶心、呕吐)、胰腺炎、消化性溃疡或穿孔等。
生物活性
倍他米松是一种糖皮质激素类固醇,具有显著的抗炎和免疫抑制特性。它对II型糖皮质激素受体具有特异亲合力,并存在于大多数脑区。
体内研究
在妊娠晚期胎羊中,倍他米松可直接收缩外围股动脉血管并减少脑血流量(CBF)。此外,在大鼠实验中发现,倍他米松能够减少NF-κB的活化及肿瘤坏死因子α和IL-1β水平上升,并诱导大脑中IL-10的表达。这些作用与疼痛阈值的变化有关。
倍他米松还能在神经损伤时注射部分减轻神经性痛觉过敏的发展,降低随后升高的脑促炎性细胞因子水平,同时促进抗炎细胞因子IL-10的表达。此外,在成年大鼠肺部中,倍他米松小幅增加胞苷酰转移酶(CT)的表达,并在神经损伤时注射部分减少了炎症性介质。
化学性质
倍他米松为白色结晶粉末,熔点231-234℃。醋酸倍他米松亦为白色结晶粉末,熔点205-208℃。它微溶于丙醇和乙醇,极难溶于氯仿或乙醚,并不溶于水。
用途
倍他米松主要用于抗炎及抗过敏治疗。适用于风湿性关节炎及各种皮肤病等临床病症。
生产方法
醋酸倍他米松通过甲醇-氯仿-水的混合物中以盐酸处理,可转换为倍他米松。
分类与毒性
倍他米松属于有毒物品,具有中毒级别,急性口服小鼠LD50大于4500毫克/公斤。该药物是可燃的,在燃烧时会产生有毒氟化物烟雾。
储运与灭火方法
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 多倍他索 16β-methyl-9α-fluoro-11β,17α-dihydroxy-1,4-pregnadiene-3,20-dione 1879-77-2 C22H29FO4 376.468 醋酸倍氟美松 betamethasone 21-acetate 987-24-6 C24H31FO6 434.505 倍他米松二丙酸酯 betamethasone dipropionate 5593-20-4 C28H37FO7 504.596 (16beta)-17,21-二羟基-16-甲基孕甾-1,4,9(11)-三烯-3,20-二酮 17α,21-dihydroxy-16β-methylpregna-1,4,9(11)-triene-3,20-dione 13504-15-9 C22H28O4 356.462 17Α-羟基-16Β-甲基孕甾-1,4,9(11)-三烯-3,20-二酮 16β-methyl-17α-hydroxy-pregna-1,4,9(11)-triene-3,20-dione 14135-32-1 C22H28O3 340.463 倍他米松环氧水解物 9β,11β-epoxy-17α,21-dihydroxy-16β-methylpregna-1,4-diene-3,20-dione 981-34-0 C22H28O5 372.461 9Β,11Β-环氧-16Β-甲基孕甾-1,4-二烯-17Α-醇-3,20-二酮 16β-methyl-9β,11β-epoxy-17α-hydroxy-1,4-pregnadiene-3,20-dione 37413-99-3 C22H28O4 356.462 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 氯倍他索 clobetasol 25122-41-2 C22H28ClFO4 410.913 醋酸倍氟美松 betamethasone 21-acetate 987-24-6 C24H31FO6 434.505 9-氟-11beta,17,21-三羟基-16beta-甲基孕甾-1,4-二烯-3,20-二酮21-(琥珀酸氢酯) betamethasone-21-hemisuccinate 27297-42-3 C26H33FO8 492.542 L-倍他米松磷酸钠杂质F 9α-fluoro-11β,17α-dihydroxy-16β-methyl-3-oxo-androsta-1,4-diene-17β-carboxylic acid 37926-75-3 C21H27FO5 378.441 倍他米松磷酸酯 betamethasone-21-phosphate 360-63-4 C22H30FO8P 472.448 9-氟-11beta,17,21-三羟基-16beta-甲基孕甾-1,4-二烯-3,20-二酮 17-丙酸酯 Betamethasone propionate 5534-13-4 C25H33FO6 448.532 倍他米松17-丁酸酯 9α-fluoro-11β,17α,21-trihydroxy-16β-methyl-1,4-pregnadiene-3,20-dione 17-butyrate 5534-14-5 C26H35FO6 462.559 倍他米松戊酸酯 betamethasone-valerate 2152-44-5 C27H37FO6 476.586 —— (11beta,16beta)-21-Ethoxy-9-fluoro-11-hydroxy-16-methyl-3,20-dioxopregna-1,4-dien-17-yl propanoate 123013-29-6 C27H37FO6 476.6 倍他米松二丙酸酯 betamethasone dipropionate 5593-20-4 C28H37FO7 504.596 倍他米松丙酸酪酸盐 (+)-9-fluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17-butyrate 21-propionate 5534-02-1 C29H39FO7 518.623 (8S,9R,10S,11S,13S,14S,16S)-9-氟-11-羟基-10,13,16-三甲基-6,7,8,11,12,14,15,16-八氢环戊烯并[a]菲-3,17-二酮 9α-fluoro-11β-hydroxy-16β-methylandrosta-1,4-diene-3,17-dione 3109-01-1 C20H25FO3 332.415 替莫贝松 11β,17α-dihydroxy-9α-fluoro-17β-<(methylthio)carbonyl>-16β-methylandrosta-1,4-dien-3-one 87116-72-1 C22H29FO4S 408.534 —— Betamethasone 17α,21-ethyl orthopropanoate 1062-09-5 C27H37FO6 476.586 倍他米松17-丙酸酯21-甲磺酸酯 betamethasone 17-propanoate 21-mesylate 15423-80-0 C26H35FO8S 526.624 —— 9α-fluoro-11β-hydroxy-16β-methyl-21-methylthio-17α-propanoyloxy-1,4-pregnadiene-3,20-dione 128291-99-6 C26H35FO5S 478.625 —— 17α-acetoxy-21-ethoxycarbonylthio-9α-fluoro-11β-hydroxy-16β-methyl-1,4-pregnadiene-3,20-dione 120815-86-3 C27H35FO7S 522.635 —— (Z)-betamethasone 20-hydroxy-17(20)-en-21-aldehyde 6762-45-4 C22H27FO4 374.452 [(8S,10S,11S,13S,14S,16S)-9-氟-11-羟基-10,13,16-三甲基-17-甲硫基羰基-3-氧代-6,7,8,11,12,14,15,16-八氢环戊烯并[a]菲-17-基]乙酸酯 17α-acetoxy-9α-fluoro-11β-hydroxy-17β-<(methylthio)carbonyl>-16β-methylandrosta-1,4-dien-3-one 79578-14-6 C24H31FO5S 450.572 可的松 Cortisone 53-06-5 C21H28O5 360.45 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:一种倍他米松17α-丙酸酯的制备方法摘要:一种倍他米松17α‑丙酸酯的制备方法,采用倍他米松与原丙酸三乙酯反应,所述的反应在在四氢呋喃溶剂中进行且采用对甲苯磺酸作为催化剂,反应完成后,不经出料,直接在同一溶剂体系下,向体系中滴加三氯化铝溶液,滴加完毕后,保温反应,反应完毕后,经过后处理工艺得到倍他米松17α‑丙酸酯。本发明在解决倍他米松17α‑丙酸酯采用二氧六环或者二甲基甲酰胺或者其他溶剂等做反应溶剂的同时,还简化了制造流程、提高了收率和纯度。公开号:CN110003299A
-
作为产物:参考文献:名称:倍他米松或地塞米松合成母液料的回收利用 方法摘要:本发明涉及一种倍他米松或地塞米松合成母液料的回收利用方法,包括步骤:从母料液提取式1所示化合物;将式1所示化合物进行20位羟基基团的保护反应,得到式2所示化合物;将式2所示化合物进行21位醛基的还原反应,得到式3所示化合物,继续进行氧化反应和水解反应,得到式4所示化合物,所述式4所示化合物即为倍他米松或地塞米松;上述回收利用方法可将倍他米松或地塞米松母液料转化成药用价值和经济效益高的倍他米松或地塞米松,具有巨大的经济效益。公开号:CN110845562B
文献信息
-
DISUBSTITUTED TRIFLUOROMETHYL PYRIMIDINONES AND THEIR USE申请人:BAYER PHARMA AKTIENGESELLSCHAFT公开号:US20160221965A1公开(公告)日:2016-08-04The present application relates to novel 2,5-disubstituted 6-(trifluoromethyl)pyrimidin-4(3H)-one derivatives, to processes for their preparation, to their use alone or in combinations for the treatment and/or prevention of diseases, and to their use for preparing medicaments for the treatment and/or prevention of diseases, in particular for treatment and/or prevention of cardiovascular, renal, inflammatory and fibrotic diseases.
-
[EN] INHIBITORS OF BRUTON'S TYROSINE KINASE<br/>[FR] INHIBITEURS DE TYROSINE KINASE DE BRUTON申请人:BIOCAD JOINT STOCK CO公开号:WO2018092047A1公开(公告)日:2018-05-24The present invention relates to a new compound of formula I: or pharmaceutically acceptable salt, solvate or stereoisomer thereof, wherein: V1 is C or N, V2 is C(R2) or N, whereby if V1 is C then V2 is N, if V1 is C then V2 is C(R2), or if V1 is N then V2 is C(R2); each n, k is independently 0, 1; each R2, R11 is independently H, D, Hal, CN, NR'R", C(O)NR'R", C1-C6 alkoxy; R3 is H, D, hydroxy, C(O)C1-C6 alkyl, C(O)C2-C6 alkenyl, C(O)C2-C6 alkynyl, C1-C6 alkyl; R4 is H, Hal, CN, CONR'R", hydroxy, C1-C6 alkyl, C1-C6 alkoxy; L is CH2, NH, O or chemical bond; R1 is selected from the group of the fragments, comprising: Fragment 1, Fragment 2, Fragment 3 each A1, A2, A3, A4 is independently CH, N, CHal; each A5, A6, A7, A8, A9 is independently C, CH or N; R5 is H, CN, Hal, CONR'R", C1-C6 alkyl, non-substituted or substituted by one or more halogens; each R' and R" is independently selected from the group, comprising H, C1-C6 alkyl, C1-C6 cycloalkyl, aryl; R6 is selected from the group: [formula II] each R7, R8, R9, R10 is independently vinyl, methylacetylenyl; Hal is CI, Br, I, F, which have properties of inhibitor of Bruton's tyrosine kinase (Btk), to pharmaceutical compositions containing such compounds, and their use as pharmaceuticals for treatment of diseases and disorder.本发明涉及一种新的化合物,其化学式为I:或其药学上可接受的盐、溶剂化合物或立体异构体,其中:V1为C或N,V2为C(R2)或N,如果V1为C,则V2为N,如果V1为C,则V2为C(R2),或者如果V1为N,则V2为C(R2);每个n,k独立地为0或1;每个R2,R11独立地为H,D,Hal,CN,NR'R",C(O)NR'R",C1-C6烷氧基;R3为H,D,羟基,C(O)C1-C6烷基,C(O)C2-C6烯基,C(O)C2-C6炔基,C1-C6烷基;R4为H,Hal,CN,CONR'R",羟基,C1-C6烷基,C1-C6烷氧基;L为CH2,NH,O或化学键;R1从包括的片段组中选择:片段1,片段2,片段3,每个A1,A2,A3,A4独立地为CH,N,CHal;每个A5,A6,A7,A8,A9独立地为C,CH或N;R5为H,CN,Hal,CONR'R",C1-C6烷基,未取代或被一个或多个卤素取代;每个R'和R"独立地从包括H,C1-C6烷基,C1-C6环烷基,芳基的组中选择;R6从组中选择:[化学式II]每个R7,R8,R9,R10独立地为乙烯基,甲基乙炔基;Hal为CI,Br,I,F,具有布鲁顿酪氨酸激酶(Btk)抑制剂的性质,以及含有这种化合物的药物组合物,以及它们作为治疗疾病和紊乱的药物的用途。
-
[EN] DIHYDROPYRROLONAPHTYRIDINONE COMPOUNDS AS INHIBITORS OF JAK<br/>[FR] COMPOSÉS DE DIHYDROPYRROLONAPHTYRIDINONE COMME INHIBITEURS DE JAK申请人:TAKEDA PHARMACEUTICAL公开号:WO2010144486A1公开(公告)日:2010-12-16Disclosed are JAK inhibitors of formula (I) where G1, R1, R2, R3, R4, R5, R6, and R7 are defined in the specification. Also disclosed are pharmaceutical compositions, kits and articles of manufacture which contain the compounds, methods and materials for making the compounds, and methods of using the compounds to treat diseases, disorders, and conditions involving the immune system and inflammation, including rheumatoid arthritis, hematological malignancies, epithelial cancers (i.e., carcinomas), and other diseases, disorders or conditions associated with JAK.揭示了式(I)的JAK抑制剂,其中G1、R1、R2、R3、R4、R5、R6和R7在规范中定义。还披露了含有这些化合物的药物组合物、试剂盒和制造物品,制备这些化合物的方法和材料,以及使用这些化合物治疗涉及免疫系统和炎症的疾病、紊乱和症状的方法,包括类风湿关节炎、血液恶性肿瘤、上皮癌(即癌症)和其他与JAK相关的疾病、紊乱或症状。
-
[EN] NOVEL COMPOUNDS AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR THE TREATMENT OF INFLAMMATORY DISORDERS<br/>[FR] NOUVEAUX COMPOSÉS ET COMPOSITIONS PHARMACEUTIQUES LES COMPRENANT POUR LE TRAITEMENT DE TROUBLES INFLAMMATOIRES申请人:GALAPAGOS NV公开号:WO2017012647A1公开(公告)日:2017-01-26The present invention discloses compounds according to Formula (I), wherein R1, R3, R4, R5, L1, and Cy are as defined herein. The present invention also provides compounds, methods for the production of said compounds of the invention, pharmaceutical compositions comprising the same and their use in allergic or inflammatory conditions, autoimmune diseases, proliferative diseases, transplantation rejection, diseases involving impairment of cartilage turnover, congenital cartilage malformations, and/or diseases associated with hypersecretion of IL6 and/or interferons. The present invention also methods for the prevention and/or treatment of the aforementioned diseases by administering a compound of the invention.本发明公开了根据式(I)的化合物,其中R1、R3、R4、R5、L1和Cy如本文所定义。本发明还提供了该发明的化合物、制备该化合物的方法、包括相同化合物的药物组合物以及它们在过敏或炎症症状、自身免疫疾病、增殖性疾病、移植排斥、涉及软骨周转障碍的疾病、先天软骨畸形和/或与IL6和/或干扰素过度分泌相关的疾病中的使用。本发明还提供了通过给予该发明的化合物来预防和/或治疗上述疾病的方法。
-
[EN] ARYL ETHER-BASE KINASE INHIBITORS<br/>[FR] INHIBITEURS DE KINASES DE TYPE ARYLÉTHER-BASE申请人:BRISTOL MYERS SQUIBB CO公开号:WO2015038112A1公开(公告)日:2015-03-19The present disclosure is generally directed to compounds which can inhibit AAK1 (adaptor associated kinase 1), compositions comprising such compounds, and methods for inhibiting AAK1.
表征谱图
-
氢谱1HNMR
-
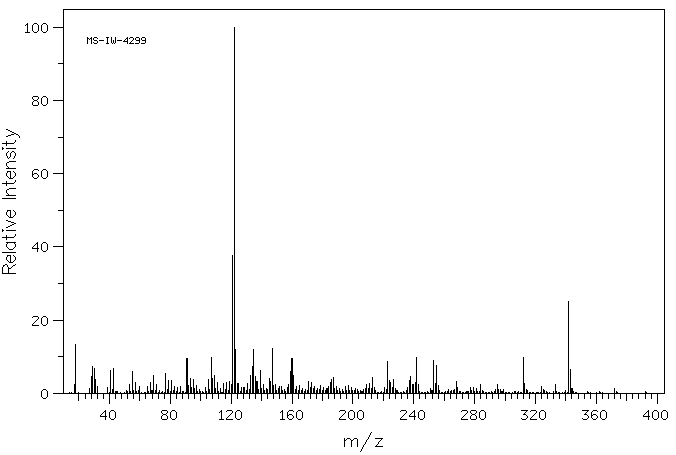
质谱MS
-
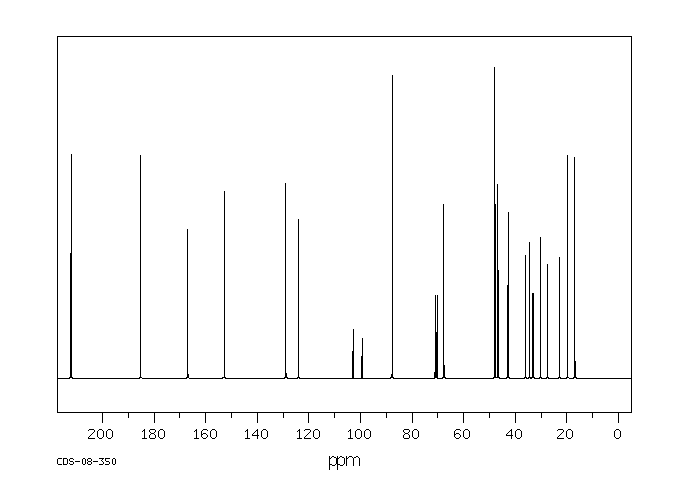
碳谱13CNMR
-
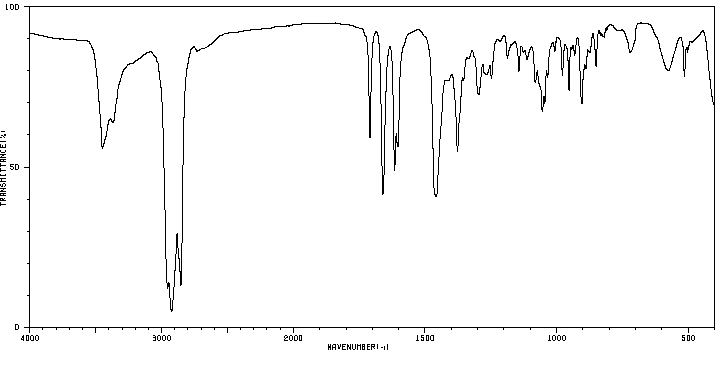
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息