双环[4.3.0]九硝基三联苯-3,6(1)-二烯 | 7603-37-4
中文名称
双环[4.3.0]九硝基三联苯-3,6(1)-二烯
中文别名
双环[4.3.0]九-3,6(1)-二烯
英文名称
2,3,4,7-tetrahydro-indene
英文别名
bicyclo<4.3.0>nona-3,6(1)-diene;bicyclo[4.3.0]nona-1(6),3-diene;bicyclo[4.3.0]nona-3,6(1)-diene;bicyclo{4.3.0}nona-3,6(1)-diene;2,3,4,7-tetrahydro-1H-indene;1,4-dihydroindane
CAS
7603-37-4
化学式
C9H12
mdl
MFCD00003776
分子量
120.194
InChiKey
PJEOOBRBALZZSL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-35 °C
-
沸点:178 °C(lit.)
-
密度:0.931 g/mL at 25 °C(lit.)
-
闪点:50 °F
-
保留指数:1038;1039;1047;1039;1040;1047;1048;1038;1059;1068;1038
-
稳定性/保质期:
避免强氧化剂、强碱和强酸。避免光、明火和高温。
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:9
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.555
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3.1
-
危险品标志:F
-
安全说明:S16,S29,S33
-
危险类别码:R11
-
WGK Germany:3
-
包装等级:II
-
危险类别:3.1
-
危险品运输编号:UN 1993 3
-
储存条件:请将药品密闭保存。
SDS
反应信息
-
作为反应物:描述:参考文献:名称:Aromatization of 1,4-dihydrobenzocycloalkenes, 1,4-dihydronaphthocycloalkenes, and related systems摘要:DOI:10.1021/jo01297a019
-
作为产物:参考文献:名称:实用且无化学选择的无氨白桦树摘要:报道了由稳定的钠分散液和可回收的15-冠-5醚介导的显着改善的,实用的和化学选择性的无氨桦木还原的新方案。各种芳族和杂芳族化合物均以优异的收率被还原。DOI:10.1021/acs.orglett.8b00891
文献信息
-
一种新型电子盐体系以及不饱和烃类化合物 的还原方法
-
Tandem aziridine ring opening-disulfide formation-reduction-Michael addition in one-pot mediated by tetrathiomolybdate作者:Devarajulu Sureshkumar、Thanikachalam Gunasundari、Srinivasan ChandrasekaranDOI:10.1016/j.tet.2015.04.003日期:2015.9A detailed study of tetrathiomolybdate mediated tandem regio- and stereoselective ring opening of aziridine, disulfide formation, reduction of disulfide bond and Michael reaction in a one-pot operation is reported. This constitutes four reactions that take place in one-pot operation. In the reaction of [BnEt3N]4MoS4 with an aziridine derived from cyclohexene and in the absence of Michael acceptor intermediates
-
Iridium(III)‐Catalyzed Intermolecular C(sp <sup>3</sup> )−H Insertion Reaction of Quinoid Carbene: A Radical Mechanism作者:Hai‐Xu Wang、Yann Richard、Qingyun Wan、Cong‐Ying Zhou、Chi‐Ming CheDOI:10.1002/anie.201911138日期:2020.1.27intermolecular C(sp3 )-H insertion reaction of a quinoid carbene (QC). The reaction was designed by harnessing the hydrogen-atom transfer (HAT) reactivity of a metal-QC species with aliphatic substrates followed by a radical rebound process to afford C-H arylation products. This methodology is efficient for the arylation of activated hydrocarbons such as 1,4-cyclohexadienes (down to 40 min reaction time, up
-
Equilibrium between homocub-1(9)-ene and homocub-9-ylidene作者:Ning Chen、Maitland Jones、Walter R. White、Matthew S. PlatzDOI:10.1021/ja00013a038日期:1991.6A combination of chemical trapping and laser flash photolysis experiments is used to demonstrate that homocub-9-ylidene and homocub-1(9)-ene are in equilibrium. The equilibrium constant can be shown to be close to unity at +20°C (0.23≤K≤4)化学捕获和激光闪光光解实验的组合用于证明 homocub-9-ylidene 和 homocub-1(9)-ene 处于平衡状态。平衡常数可以显示为在 +20°C 时接近 1 (0.23≤K≤4)
-
1,4‐Dehydrogenation with a Two‐Coordinate Cyclic (Alkyl)(amino)silylene作者:Taichi Koike、Tomoyuki Kosai、Takeaki IwamotoDOI:10.1002/chem.201901407日期:2019.7.11Cyclic (alkyl)(amino)silylene (CAASi) 1 has been found to successfully dehydrogenate 1,4‐dihydroaromatic compounds containing various substituents to afford the corresponding aromatic compounds. The observed high substrate generality proves 1 to be a potential 1,4‐dehydrogenation reagent for organic compounds. For the reaction with 9,10‐dimethyl‐9,10‐dihydroanthracene, silylene 1 activated not only
表征谱图
-
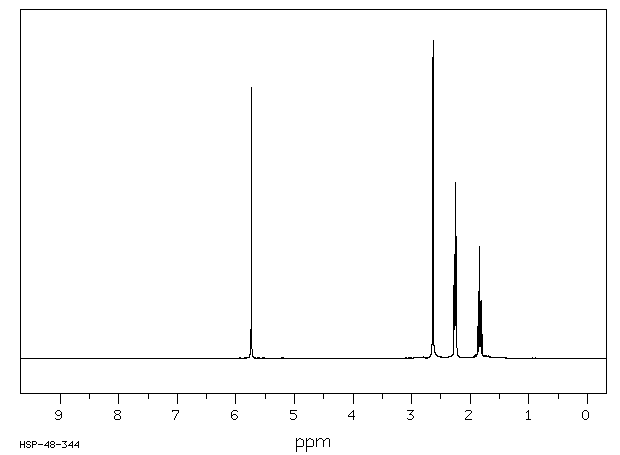
氢谱1HNMR
-
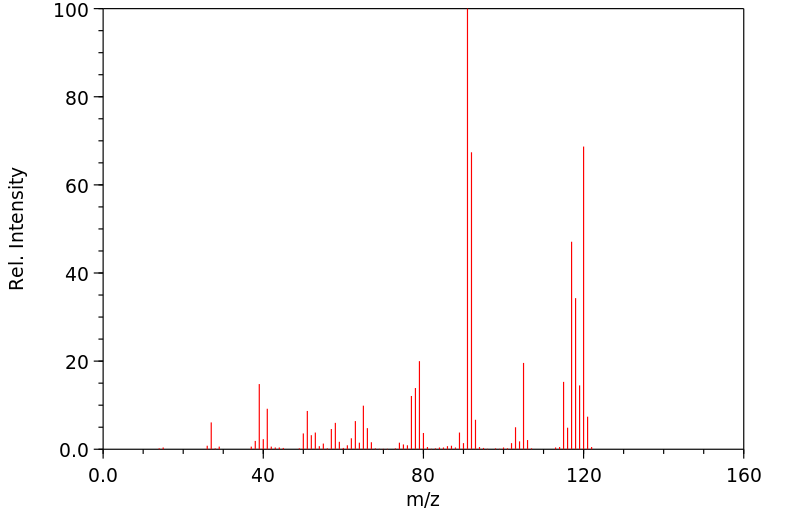
质谱MS
-
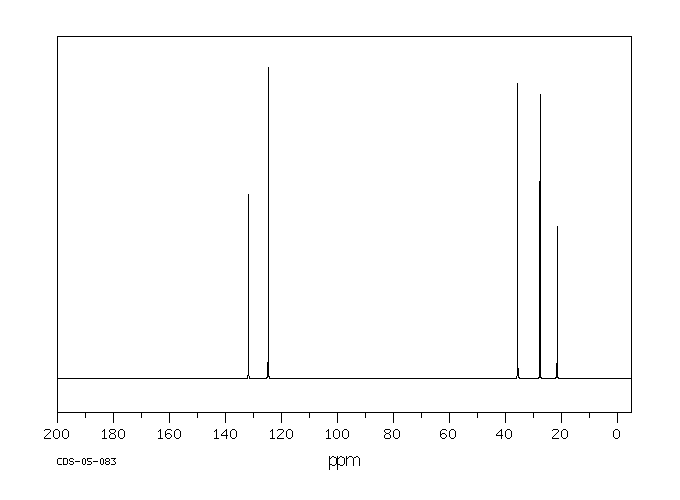
碳谱13CNMR
-
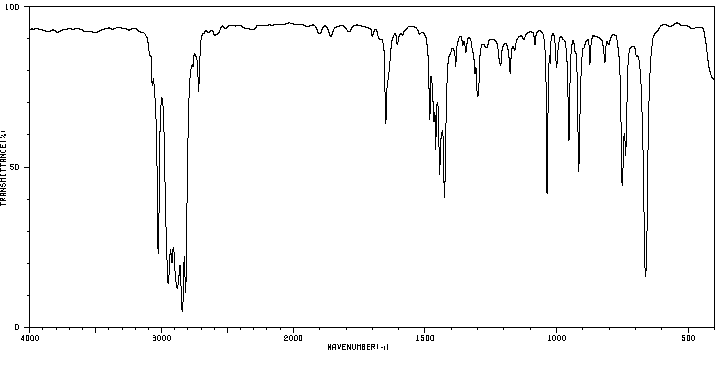
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷