螺[2.5]辛烷 | 185-65-9
中文名称
螺[2.5]辛烷
中文别名
——
英文名称
spiro[2.5]octane
英文别名
spiro<2.5>octan;spiro<2.5>octane;Spiro<<2.5>octan
CAS
185-65-9
化学式
C8H14
mdl
MFCD00060829
分子量
110.199
InChiKey
FOEYMRPOKBCNCR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:125.5 °C
-
密度:0.8282 g/cm3
计算性质
-
辛醇/水分配系数(LogP):3.7
-
重原子数:8
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 螺[2.5]-6-辛酮 spiro[2.5]octan-6-one 15811-21-9 C8H12O 124.183 1,1-二甲基环己烷 1,1-dimethylcyclohexane 590-66-9 C8H16 112.215 6-羟基螺[2.5]辛烷 spiro[2.5]octan-6-ol 22428-83-7 C8H14O 126.199 螺[2.5]-5-辛酮 spiro[2.5]octan-5-one 25308-67-2 C8H12O 124.183 乙基环己烷 ethyl-cyclohexane 1678-91-7 C8H16 112.215 5-羟基螺[2.5]辛烷 5-hydroxyspiro[2.5]octane 7647-61-2 C8H14O 126.199
反应信息
-
作为反应物:描述:参考文献:名称:环丙烷化环辛烷的直接氧化作为多环环丙基酮的合成方法摘要:在氧化条件下研究了一系列含有环丙烷部分 1,2-与环辛烷环缩合或螺环稠合的多环烃。四种氧化系统(SiO2 上的 O3、源自三氟丙酮的二环氧乙烷、CrO3 和 RuO4,原位生成)被用来评估和比较它们的反应性和实用性。发现 RuO4 是最好的,考虑到它的氧化能力和简单的制备程序。找到了多环烃特定氧化的条件。获得了含有环丙基羰基部分的新型环辛烷衍生物。DOI:10.1002/ejoc.201701671
-
作为产物:描述:参考文献:名称:Shortridge et al., Journal of the American Chemical Society, 1948, vol. 70, p. 948摘要:DOI:
文献信息
-
Combined Effects on Selectivity in Fe-Catalyzed Methylene Oxidation作者:Mark S. Chen、M. Christina WhiteDOI:10.1126/science.1183602日期:2010.1.29in organic molecules. Methylene C–H bonds are among the most difficult chemical bonds to selectively functionalize because of their abundance in organic structures and inertness to most chemical reagents. Their selective oxidations in biosynthetic pathways underscore the power of such reactions for streamlining the synthesis of molecules with complex oxygenation patterns. We report that an iron catalyst二级选择性有机分子主要由亚甲基(二级)CH2 基团的环和链组成,间歇性地装饰有氧或氮中心以及连接处更重取代的碳。如果沿着框架的任何特定亚甲基中的 C-H 键可以作为选择性修饰的目标,那么合成转化将是最有效的。然而,在大多数情况下,这些碳中心被证明非常难以区分用于反应目的。Chen 和 White (p. 566) 现在表明,铁催化剂可以引导过氧化物优先氧化一系列复杂分子中的特定二级 C-H 键,并具有合理的效率。观察到的选择性遵循与目标位点的电子和空间环境相关的可预测趋势。铁催化剂显示出对有机分子中二级 C-H 键氧化的选择性。亚甲基 C-H 键是最难选择性官能化的化学键之一,因为它们具有丰富的有机结构和对大多数化学试剂的惰性。它们在生物合成途径中的选择性氧化强调了这种反应在简化具有复杂氧化模式的分子合成方面的能力。我们报告说,铁催化剂可以在不同的天然产物环境中实现亚甲基 C-H 键氧化,
-
Dihalocarbene Insertion Reactions into C−H Bonds of Compounds Containing Small Rings: Mechanisms and Regio- and Stereoselectivities<sup>,</sup>作者:Udo H. Brinker、Guoying Lin、Linxiao Xu、William B. Smith、Jean-Luc MieussetDOI:10.1021/jo7013356日期:2007.10.1products are formed as the major isomers. In some compounds cyclopropane rings “activate” adjacent carbon−hydrogen bonds, whereas other systems containing three-membered rings do not. Moreover, the influence of various substituents (methyl, geminal dimethyl, phenyl, methoxy, and ethoxy) attached to bicyclo[3.1.0]hexane and bicyclo[4.1.0]heptane in dihalocarbene reactions has been studied. The findings据报道,新型二氯和二溴卡宾插入到邻近环丙烷环的碳氢键中的插入反应。发现与双环[4.1.0]庚烷的反应中形成的主要异构体是由于插入到三元环的碳氢键内。在双环的反应[3.1.0]己烷,然而,外形成了二卤卡宾插入产物作为主要异构体。在某些化合物中,环丙烷环会“激活”相邻的碳氢键,而其他包含三元环的系统则不会。此外,已经研究了在二卤卡宾反应中连接到双环[3.1.0]己烷和双环[4.1.0]庚烷的各种取代基(甲基,双甲基二甲基,苯基,甲氧基和乙氧基)的影响。可以用环丙烷环的沃尔什轨道和α碳氢键的最大轨道重叠概念来解释这一发现。与之形成鲜明对比的是,观察到在双环[4.2.0]辛烷中选择性插入环丁烷环的叔碳氢键中。
-
Esr studies of the ring opening of cyclopropane radical cations in freon matrices作者:Xue-Zhi Qin、Francon WilliamsDOI:10.1016/s0040-4020(01)88093-5日期:1986.1matrix, however, the ring-closed radical cations initially formed at 77 K undergo ring opening between 83 and 110 K, the more highly substituted radical cations requiring a higher temperature for this transformation. The ring-closed radical cations are 2A1 species for C2v symmetry, the most substituted cyclopropane C-C bond being elongated with the spin density largely confined to the basal carbons环丙烷及其几种甲基衍生物的自由基阳离子是在77 K氟利昂基质中通过对母体化合物的稀溶液进行γ射线辐照后通过ESR光谱表征的。在CFCl 3,CF 3 CCl 3和CF 2中ClCCl 3,矩阵,仅闭环物种在可访问的温度范围达到通常观察到CA 160 K.在CFCl 2 CF 2但是,Cl基体中,最初在77 K时形成的闭环自由基阳离子的开环在83和110 K之间,取代度更高的自由基阳离子需要较高的温度才能进行该转化。对于C 2v对称性,闭环的自由基阳离子为2 A 1物种,取代度最高的环丙烷CC键被拉长,其自旋密度在很大程度上以面对面(90°,90°)结构限制在基础碳上。在开环的自由基阳离子中,随着闭环物种弱化的CC键断裂,自由基中心位于最取代的碳原子上。已确定开环物质的自由基构象,RCH 2 CH 2·如预期的在自由基位点处存在α-甲基取代基,由具有二等分构型的环丙烷产生的中心被遮盖,而由1
-
Electroorganic Chemistry XVIII Anodic Oxidation of Substituted Cyclopropanes作者:Tatsuya Shono、Yoshihiro MatsumuraDOI:10.1246/bcsj.48.2861日期:1975.10The anodic oxidations of polyalkyl-substituted cyclopropanes (1–3) and spiro[2.n]alkanes (4–6) were carried out in methanol. Monomethoxyolefin and dimethoxy compound resulted from the selective cleavage at the most substituted carbon–carbon bond. The decrease in the oxidation potential brought about by the substitution of the one methyl group was observed to be about 0.3 V vs. SCE. The analysis of
-
Aluminum chloride catalyzed hydrosilylation of cyclopropanes with chlorodimethylsilane作者:Shigeru Nagahara、Takashi Yamakawa、Hisashi YamamotoDOI:10.1016/s0040-4039(01)00925-x日期:2001.7Aluminum chloride can be utilized as an active catalyst for the highly regioselective hydrosilylation of 1-alkyl, 1-aryl, 1,1-dialkyl, and cyclic cyclopropanes with chlorodimethylsilane in hexane at room temperature.
表征谱图
-
氢谱1HNMR
-
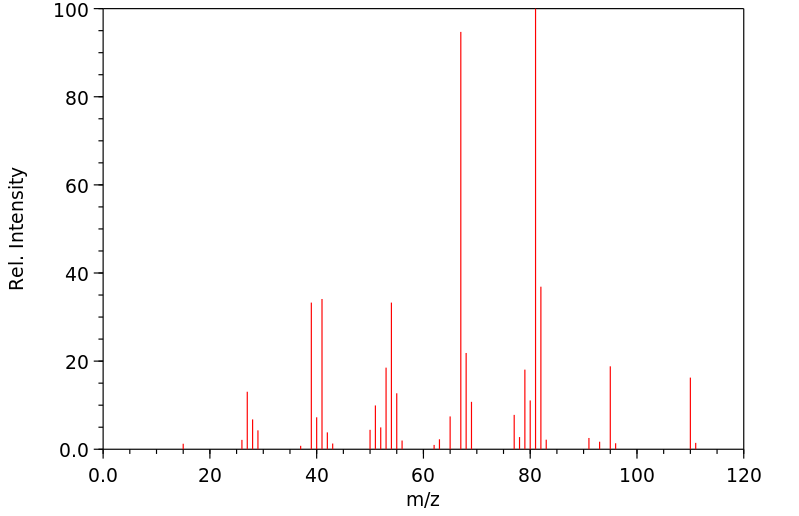
质谱MS
-
碳谱13CNMR
-
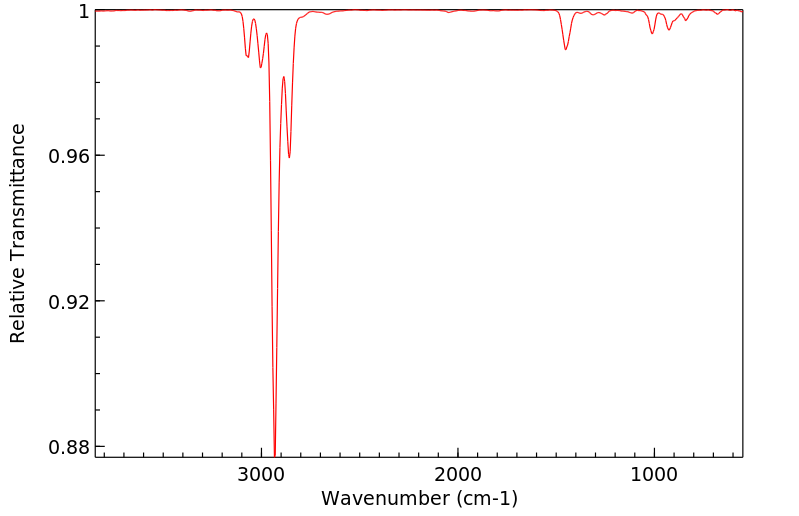
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
降冰片烯
金刚烷-D16
金刚烷
螺戊烷
螺二環己烷
螺[5.6]十二烷
螺[5.5]十一碳-4-烯
螺[5.2]辛-2-烯
螺[4.5]癸烷
螺[4.4]壬-8-烯
螺[3.4]辛烷
螺[3.4]辛-7-烯
螺[3.3]庚-2,5-二烯
螺[2.5]辛烷
螺[2.5]辛-7-烯
螺[2.5]辛-5,7-二烯
螺[2.4]庚-4,6-二烯
螺[2.4]庚-1-烯
螺[2.3]己-1-烯
螺[2.2]戊-1-烯
螺<二环<2.2.2>辛-5-烯-2,1'-环丙烷>
螺<4.4>壬-1,3,7-三烯
螺<4.4>壬-1,3,6,8-四烯
螺(4.4)壬烷
螺(4.4)壬-1,3-二烯
螺(3.4)辛-5,7-二烯
trans-perhydroazulene
萘烷
萘,1,2,3,4,4a,8a-六氢-,顺-
美罗培南中间体F9
篮烷
立方烷
氨基甲硫酸,二甲基-,O,O-(3,3-二甲基1,1-联苯基-2,2-二基)酯
棱晶烷
杜瓦苯
新戊基-1金刚烷
抗氧化剂TH-CPL
庚搭烯
四螺[2.0.2:0.2:0.2:0]十二烷
四螺[2.0.0.0.2.1.1.1]十一烷,顺-
四环己基铅
四环[5.3.0.0<2,6>.0<3,10>]癸-4,8-二烯
四环[5.3.0.0(2,6).0(3,10)]癸烷
四环[4.4.0.02,10.03,7]癸-4,8-二烯
四环[4.2.2.26,5.01,6]十二烷
四环[4.2.0.02,5.03,8]辛烷
四环[3.3.0.02,4.03,6]辛-7-烯
四环<5.3.1.02,6.04,9>十一烷
四环(8.2.2.22,5.26,9)十八碳-1,5,9-三烯
四环(4.1.0.0(2,4).0(3,5))庚烷