对氨基苯乙腈 | 3544-25-0
中文名称
对氨基苯乙腈
中文别名
4-氨基苯乙腈;4-氨基苯基乙腈;對胺苯乙腈;4-氨基苄氰
英文名称
p-aminophenylacetonitrile
英文别名
4-amino-benzeneacetonitrile;2-(4-aminophenyl)acetonitrile;4-aminobenzyl cyanide;4-aminophenylacetonitrile
CAS
3544-25-0
化学式
C8H8N2
mdl
MFCD00007912
分子量
132.165
InChiKey
YCWRFIYBUQBHJI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:45-48 °C(lit.)
-
沸点:312 °C(lit.)
-
密度:1.4747 (rough estimate)
-
闪点:>230 °F
-
溶解度:溶于甲醇
-
暴露限值:NIOSH: IDLH 25 mg/m3
-
稳定性/保质期:
常温常压下稳定,为白色或淡黄色结晶。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:49.8
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险等级:6.1
-
安全说明:S36,S36/37
-
危险品运输编号:3276
-
WGK Germany:3
-
海关编码:2926909090
-
危险类别:6.1
-
危险品标志:Xn
-
危险类别码:R20/21/22
-
包装等级:III
-
危险性防范说明:P501,P261,P270,P271,P264,P280,P361+P364,P301+P310+P330,P302+P352+P312,P304+P340+P311,P403+P233,P405
-
危险性描述:H301,H311,H331
-
储存条件:常温下应存于避光、通风且干燥的地方,并密封保存。
SDS
| Name: | 4-Aminobenzyl cyanide 99% Material Safety Data Sheet |
| Synonym: | 4-Aminophenylacetonitrile; 4-Aminobenzyl cyanide |
| CAS: | 3544-25-0 |
Synonym:4-Aminophenylacetonitrile; 4-Aminobenzyl cyanide
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 3544-25-0 | 4-Aminobenzyl cyanide | 99 | 222-587-4 |
Risk Phrases: 20/21/22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful by inhalation, in contact with skin and if swallowed.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
May be metabolized to cyanide which in turn acts by inhibiting cytochrome oxidase impairing cellular respiration. Chronic exposure to cyanide solutions may lead to the development of a "cyanide" rash, characterized by itching, and by macular, papular, and vesicular eruptions, and may be accompanied by secondary infections. Exposure to small amounts of cyanide compounds over long periods of time is reported to cause loss of appetite, headache, weakness, nausea, dizziness, and symptoms of irritation of the upper respiratory tract and eyes.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Get medical aid immediately. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
If inhaled, remove to fresh air. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Combustion generates toxic fumes. This material in sufficient quantity and reduced particle size is capable of creating a dust explosion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Wash hands before eating. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Use only with adequate ventilation. Avoid breathing dust.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 3544-25-0: United States OSHA: 5 mg/m3 TWA (listed under Cyanide anion).
Germany: (listed as cyanide anion): Skin absorber Netherlands: (listed as cyanide anion): 5 mg/m3 MAC (as CN) Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: yellow to brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 312 deg C @ 760 mm Hg
Freezing/Melting Point: 44-48 deg C
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: slightly soluble
Specific Gravity/Density:
Molecular Formula: C8H8N2
Molecular Weight: 132.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Dust generation.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong reducing agents, strong acids, and strong bases.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, cyanides.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 3544-25-0 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Aminobenzyl cyanide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Ecotoxicity:
Bacteria: Phytobacterium phosphoreum: EC50 = 0.36-0.40 mg/L; 5,15,30 minutes; Microtox test; 15 degrees C
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: NITRILES, SOLID, TOXIC, N.O.S.*
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
IMO
Shipping Name: Nitriles, Toxic, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing Group: III
RID/ADR
Shipping Name: Nitriles, Toxic, N.O.S.
Hazard Class: 6.1
UN Number: 3276
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 20/21/22 Harmful by inhalation, in contact with
skin and if swallowed.
Safety Phrases:
S 36/37 Wear suitable protective clothing and
gloves.
WGK (Water Danger/Protection)
CAS# 3544-25-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 3544-25-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 3544-25-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 苯乙腈 phenylacetonitrile 140-29-4 C8H7N 117.15 对硝基苯乙腈 4-Nitrophenylacetonitrile 555-21-5 C8H6N2O2 162.148 —— (4-amino-phenyl)-thioacetic acid amide —— C8H10N2S 166.247 对溴苯乙腈 4-Bromophenylacetonitrile 16532-79-9 C8H6BrN 196.046 对氯苯乙腈 p-chlorobenzyl cyanide 140-53-4 C8H6ClN 151.595 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(cyanomethyl)phenylhydrazine 57411-91-3 C8H9N3 147.18 —— [4-(methylamino)phenyl]acetonitrile 68384-47-4 C9H10N2 146.192 2-(4-氨基苯基)丙腈 2-(4-aminophenyl)propanenitrile 28694-90-8 C9H10N2 146.192 —— (4-dimethylamino-phenyl)-acetonitrile 34906-70-2 C10H12N2 160.219 —— 4-(N-formylamino) benzyl cyanide 216063-76-2 C9H8N2O 160.175 2-(4-氨基苯)乙胺 p-Aminophenethylamine 13472-00-9 C8H12N2 136.197 —— 1-azido-4-cyanomethylbenzene —— C8H6N4 158.162 —— α-Cyan-4-tolyl-isothiocyanat 22676-71-7 C9H6N2S 174.226 —— 2-{4-[(cyanomethyl)amino]phenyl}acetonitrile —— C10H9N3 171.202 4-(2-二甲基氨基乙基)-苯胺 4-[2-(dimethylamino)ethyl]aniline 5636-52-2 C10H16N2 164.25 —— 4-pentylaminobenzeneacetonitrile —— C13H18N2 202.299 —— 4-(2-aminoethyl)-N-methylaniline 211103-69-4 C9H14N2 150.224 4-乙酰氨基苯乙腈 N-(4-cyanomethyl-phenyl)-acetamide 25025-06-3 C10H10N2O 174.202 —— (4-cyanomethyl-phenyl)-thiourea 99359-02-1 C9H9N3S 191.257 [4-(二乙基氨基)苯基]乙腈 2-((4-diethylamino)phenyl)acetonitrile 806605-05-0 C12H16N2 188.272 —— 4-((4-(cyanomethyl)phenyl)amino)butanenitrile —— C12H13N3 199.255 乙烷,三氯氟- p-toluidine 106-49-0 C7H9N 107.155 —— 2-(4-amino-phenyl)-acetamidine —— C8H11N3 149.195 对氨基苯乙酸 4-aminophenylacetic acid 1197-55-3 C8H9NO2 151.165 2-(4-氨基苯基)乙酰胺 2-(4-aminophenyl)acetamide 6633-76-7 C8H10N2O 150.18 —— 2-(4-amino-3-chlorophenyl)acetonitrile 80199-02-6 C8H7ClN2 166.61 —— 4-Amino-N,N-diethyl-phenaethylamin 1665-60-7 C12H20N2 192.304 2-(对二甲基氨基苯基)乙胺 4-(2-aminoethyl)-N,N-dimethylaniline 52632-05-0 C10H16N2 164.25 (4-氨基-3-溴苯基)乙腈 2-(4-amino-3-bromophenyl)acetonitrile 882855-96-1 C8H7BrN2 211.061 2-氯-N-[4-(氰基甲基)苯基]乙酰胺 2-chloro-N-(4-(cyanomethyl)phenyl)acetamide 90772-87-5 C10H9ClN2O 208.647 —— N-(4-cyanomethylphenyl)methanesulfonamide 33893-37-7 C9H10N2O2S 210.257 —— 4-(2,3-Epoxypropyl-amino)benzylcyanide 159873-03-7 C11H12N2O 188.229 [5-[(e)-2-(2-氯-3,4-二甲氧基苯基)乙烯基] -2-氧代-1,3,4-恶二唑-3(2h)-基]乙酸 4-amino-3,5-dichlorobenzylcyanide 109421-34-3 C8H6Cl2N2 201.055 对氨基乙酸苯甲酯 methyl p-aminophenylacetate 39552-81-3 C9H11NO2 165.192 —— 2-(4-(pyrrolidin-1-yl)phenyl)acetonitrile —— C12H14N2 186.257 - 1
- 2
- 3
反应信息
-
作为反应物:描述:对氨基苯乙腈 在 N,N-二异丙基乙胺 、 N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate 、 肼 作用下, 以 N,N-二甲基甲酰胺-d7 、 乙腈 为溶剂, 反应 28.0h, 生成 米拉贝隆参考文献:名称:Mirabegron合成新方法的研究摘要:Mirabegron是一种肌肉松弛药,用于治疗膀胱过度活动症。现有的米拉贝隆合成方法生产的中间产物4-(2-((苯乙基氨基)乙基)苯胺,使最终产品的纯化过程复杂化。在这项研究中,我们设计了一种低成本的原料用于米拉贝隆的新合成路线,并以99.6%的纯度和61%的总收率生产米拉贝隆。特别是,这种新的合成路线没有产生副产物4-(2-(苯乙氨基)乙基)苯胺,这大大简化了产物纯化过程。DOI:10.1002/jhet.2871
-
作为产物:描述:4,4'-di(cyanomethyl)azobenzene 在 palladium on activated charcoal aminomethylpolystyrene-supported formate 作用下, 以 甲醇 为溶剂, 反应 5.0h, 以90%的产率得到对氨基苯乙腈参考文献:名称:Palladium-catalyzed simple and efficient hydrogenative cleavage of azo compounds using recyclable polymer-supported formate摘要:钯催化室温下使用可回收的聚合物支持的甲酸根转移氢化偶氮化合物,产生对应的胺类产物,收率极高(88%至98%)。该方法被发现在选择性上高度便利,对许多其他官能团如卤素、烯烃、腈、酮、酰胺、甲氧基和羟基具有选择性。关键词:偶氮化合物、催化转移氢化、聚合物支持的甲酸根、10% Pd-C、胺类。DOI:10.1139/v05-071
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Highly chemoselective reduction of nitroarenes over non-noble metal nickel-molybdenum oxide catalysts作者:Haigen Huang、Xueguang Wang、Xu Li、Chenju Chen、Xiujing Zou、Weizhong Ding、Xionggang LuDOI:10.1039/c6gc03141b日期:——Chemoselective reduction of nitroarenes is an important transformation for the production of arylamines, which are the primary intermediates in the synthesis of pharmaceuticals, agrochemicals and dyes. Heterogeneous non-noble metal nickel-molybdenum...
-
A Facile Reduction of Nitroarenes to Anilines Using FeCl<sub>3 </sub>· 6H<sub>2</sub>O/Indium作者:Byung Woo Yoo、Jin Woo Choi、Sun Kyun Hwang、Dong Yoon Kim、Heung Soo Baek、Kyung Il Choi、Joong Hyup KimDOI:10.1081/scc-120022471日期:2003.9.1Abstract Reduction of a variety of nitroaromatic compounds to the corresponding anilines occurs chemoselectively in high yields upon treatment with a new reduction system consisting of FeCl3 · 6H2O/indium in aqueous methanol.
-
一种芳基伯胺钌配合物、制备方法及其用途
-
[3+1+1] type cyclization of ClCF<sub>2</sub>COONa for the assembly of imidazoles and tetrazoles <i>via in situ</i> generated isocyanides作者:Ya Wang、Yao Zhou、Qiuling SongDOI:10.1039/d0cc01919d日期:——A facile synthesis of imidazoles and tetrazoles via [3+1+1] type cyclization of ClCF2COONa is developed. A diverse array of imidazoles and tetrazoles were obtained in decent yields via isocyanide intermediates. Notably, this is the first example of the cycloaddition of in situ generated isocyanides.
表征谱图
-
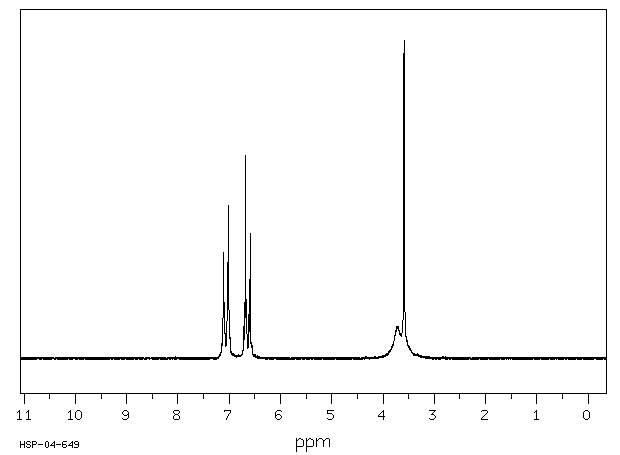
氢谱1HNMR
-
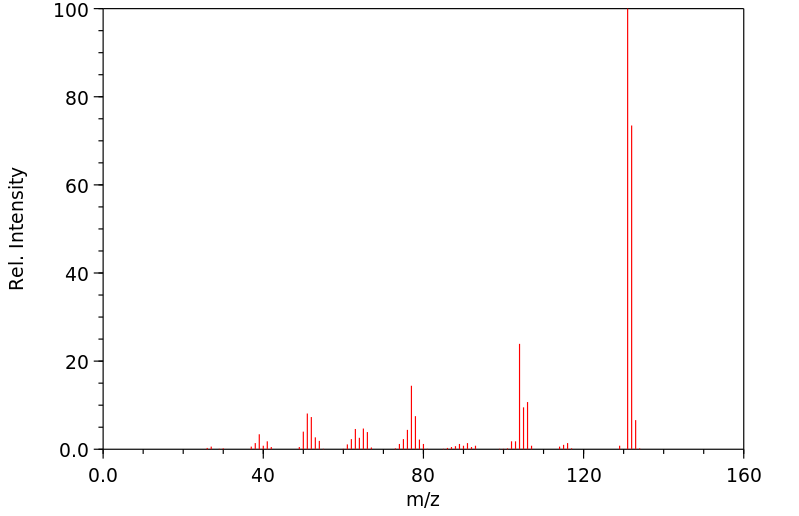
质谱MS
-
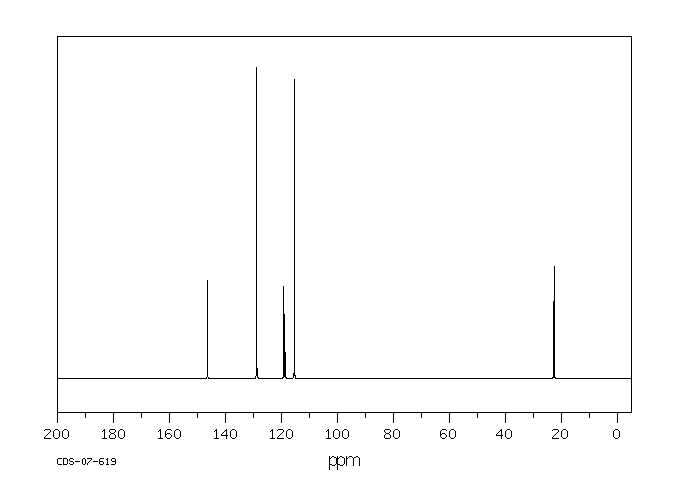
碳谱13CNMR
-
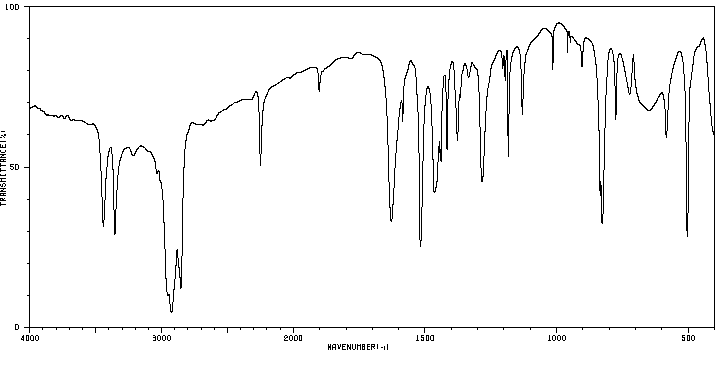
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫