帕潘立酮 | 144598-75-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:158-160°C
-
沸点:612.3±65.0 °C(Predicted)
-
密度:1.45±0.1 g/cm3(Predicted)
-
闪点:9℃
-
溶解度:DMSO:可溶,2mg/mL,澄清(加热)
-
物理描述:Solid
-
颜色/状态:Crystals from 2-propanol
-
蒸汽压力:1.3X10-15 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
解离常数:pKa = 8.76 (est)
-
碰撞截面:209.9 Ų [M+H]+ [CCS Type: TW, Method: Major Mix IMS/Tof Calibration Kit (Waters)]
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:31
-
可旋转键数:4
-
环数:5.0
-
sp3杂化的碳原子比例:0.521
-
拓扑面积:82.2
-
氢给体数:1
-
氢受体数:7
ADMET
安全信息
-
危险等级:6.1
-
危险品标志:T
-
安全说明:S45
-
危险类别码:R25
-
WGK Germany:3
-
海关编码:29349990
-
RTECS号:UV1164720
-
危险品运输编号:UN 2811 6.1/PG 3
-
储存条件:室温
SDS
模块 1. 化学品
1.1 产品标识符
: Paliperidone
产品名称
1.2 鉴别的其他方法
3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-4H-
pyrido[1,2-a]pyrimidin-4-one
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
急性毒性, 经口 (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 危险
危险申明
H301 吞咽会中毒
警告申明
预防措施
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
事故响应
P301 + P310 如果吞下去了: 立即呼救解毒中心或医生。
P321 具体处置(见本标签上提供的急救指导)。
P330 漱口。
安全储存
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 3-[2-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-
别名
tetrahydro-9-hydroxy-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one
: C23H27FN4O3
分子式
: 426.48 g/mol
分子量
组分 浓度或浓度范围
Paliperidone
<=100%
化学文摘登记号(CAS 144598-75-4
No.)
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 立即将患者送往医院。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
戴呼吸罩。 避免粉尘生成。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
避免吸入粉尘。
6.2 环境保护措施
如能确保安全,可采取措施防止进一步的泄漏或溢出。 不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
收集和处置时不要产生粉尘。 扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免形成粉尘和气溶胶。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
避免与皮肤、眼睛和衣服接触。 休息前和操作本品后立即洗手。
个体防护设备
眼/面保护
面罩與安全眼鏡请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
飞溅保护
物料: 丁腈橡胶
最小的层厚度 0.11 mm
溶剂渗透时间: 480 min
测试过的物质Dermatril® (KCL 740 / Z677272, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能微粒防毒面具N99型(US)
或P2型(EN
143)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防毒
面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 大鼠 - 65 mg/kg
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 误吞会中毒。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: UV1164720
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: 2811 国际海运危规: 2811 国际空运危规: 2811
14.2 联合国运输名称
欧洲陆运危规: TOXIC SOLID, ORGANIC, N.O.S. (Paliperidone)
国际海运危规: TOXIC SOLID, ORGANIC, N.O.S. (Paliperidone)
国际空运危规: Toxic solid, organic, n.o.s. (Paliperidone)
14.3 运输危险类别
欧洲陆运危规: 6.1 国际海运危规: 6.1 国际空运危规: 6.1
14.4 包裹组
欧洲陆运危规: III 国际海运危规: III 国际空运危规: III
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
上述信息视为正确,但不包含所有的信息,仅作为指引使用。本文件中的信息是基于我们目前所知,就正
确的安全提示来说适用于本品。该信息不代表对此产品性质的保证。
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
帕潘立酮是一种化学物质,是利培酮的主要活性代谢产物,属于非典型抗精神病药物。广泛应用于分子生物学、药理学等科研领域,为苯并异噁唑衍生物,具有多种生物活性。
生物活性Paliperidone作为Risperidone的主要活性代谢物,是一种高效的5-HT2A和D2受体拮抗剂,用于治疗精神分裂症。其体内体外研究显示,它能够显著增加Rb23和DOX在细胞内的累积,并对神经元放电率和攻击行为产生影响。
靶点| Target | Value |
|---|---|
| D2受体 |
Paliperidone在低浓度(10和50 μM)下,能够有效地作用于Aβ(25-35) 和MPP+(+),并仅保护SH-SY5Y免受过氧化氢的损害。此外,它还能显著减少各种压力诱导的细胞减少,并且在最高剂量下增强多巴胺毒性,是唯一一种能增加细胞活性(8.1%)的药物。
体内研究Paliperidone能够恢复大鼠前额叶皮质基底层细胞外的谷氨酸盐水平,防止急性MK-801诱导的细胞外谷氨酸盐增加。与Escitalopram联合给药可恢复NE神经元放电率的抑制和神经元突发放电的百分比,并在有效剂量下以剂量依赖的方式减少咬合和攻击行为。
用途上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 利培酮 Risperidone 106266-06-2 C23H27FN4O2 410.491 帕潘立酮棕榈酸酯 paliperidone palmitate 199739-10-1 C39H57FN4O4 664.904 —— 4-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)-4-oxobutanoic acid 1289120-87-1 C27H31FN4O6 526.565 —— 3-[2-[4-(6-Fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-9-(oxan-2-yloxy)-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one 1109182-35-5 C28H35FN4O4 510.609 9-氧代利司哌酮 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-9-oxo-2-methyl-6,7,8,9-tetrahydro-4H-pyrido-[1,2-a]pyrimidin-4-one 1189516-65-1 C23H25FN4O3 424.475 —— 3-(2-{4-((2,4-difluorophenyl)hydroxyiminomethyl)piperidin-1-yl}ethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-pyrido[1,2-a]pyrimidin-4-one 1141761-80-9 C23H28F2N4O3 446.497 3-(2-氯乙基)-6,7,8,9-四氢-9-羟基-2-甲基-4H-吡啶并[1,2-a]嘧啶-4-酮 3-(2-chloroethyl)-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one 130049-82-0 C11H15ClN2O2 242.705 帕潘立酮杂质7 3-(2-chloroethyl)-6,7,8,9-tetrahydro-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidine-4-one palmitate ester 1415488-05-9 C27H45ClN2O3 481.119 帕潘立酮杂质 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-9-hydroxy-2-methyl-4H-pyrido-[1,2-a]pyrimidin-4-one 766485-15-8 C23H23FN4O3 422.459 —— 3-{2-[4-(6-fluoro-benzoisooxazol-3-yl)-piperidin-1-yl]-ethyl}-9-benzyloxy-2-methyl-pyrido[1,2-a]pyrimidin-4-one 1056034-13-9 C30H29FN4O3 512.584 帕潘立酮杂质 3-(2-chloroethyl)-9-oxo-2-methyl-6,7,8,9-tetrahydro-4H-pyrido-[1,2-a]pyrimidin-4-one 1138463-56-5 C11H13ClN2O2 240.689 —— 3-(2-chloroethyl)-6,7,8,9,-tetrahydro-9hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one —— C10H13ClN2O2 228.678 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 9-(3-aminopropoxy)-3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one 1570061-79-8 C26H34FN5O3 483.586 —— 6-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)hexanoic acid 1570061-81-2 C29H37FN4O5 540.635 —— N-(3-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)propyl)pivalamide 1570061-78-7 C31H42FN5O4 567.704 帕潘立酮棕榈酸酯 paliperidone palmitate 199739-10-1 C39H57FN4O4 664.904 十八酸3-[2-[4-(6-氟-1,2-苯并恶唑-3-基)-1-哌啶基]乙基]-6,7,8,9-四氢-2-甲基-4-氧代-4H-吡啶并[1,2-a]嘧啶-9-酯 paliperidone stearate 1172995-13-9 C41H61FN4O4 692.958 帕利哌酮十四酸酯 paliperidone myristate 1172995-11-7 C37H53FN4O4 636.851 —— 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-9-((6-oxo-6-(piperazin-1-yl)hexyl)oxy)-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one 1570061-83-4 C33H45FN6O4 608.757 —— 4-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)-4-oxobutanoic acid 1289120-87-1 C27H31FN4O6 526.565 —— paliperidone oleate 1416127-88-2 C41H59FN4O4 690.942 —— 4-(4-(6-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)hexanoyl)piperazin-1-yl)-4-oxobutanoic acid 1570061-84-5 C37H49FN6O7 708.83 —— 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl 3-pivalamidopropanoate 1570061-76-5 C31H40FN5O5 581.688 —— 3-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)-N-(3-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)propyl)propanamide 1570061-80-1 C33H39FN6O6 634.708 —— tert-butyl 4-(6-((3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl)oxy)hexanoyl)piperazine-1-carboxylate 1570061-82-3 C38H53FN6O6 708.874 —— 3-(2-(4-(6-fluorobenzo[d]isoxazol-3-yl)piperidin-1-yl)ethyl)-2-methyl-4-oxo-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-9-yl 3-(3-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)propanamido)propanoate 1570061-77-6 C33H37FN6O7 648.691 9-氧代利司哌酮 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)piperidin-1-yl]ethyl]-9-oxo-2-methyl-6,7,8,9-tetrahydro-4H-pyrido-[1,2-a]pyrimidin-4-one 1189516-65-1 C23H25FN4O3 424.475 —— 9-Amino-3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7-dihydropyrido[1,2-a]pyrimidin-4-one 1301724-93-5 C23H26FN5O2 423.49 帕利哌酮开环杂质 3-{2-[4-(4-fluoro-2-hydroxybenzoyl)piperidin-1-yl]ethyl}-9-hydroxy-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one 152542-03-5 C23H28FN3O4 429.491 3-乙基-6,7,8,9-四氢-9-羟基-2-甲基-4H-吡啶并[1,2-a]嘧啶-4-酮 3-ethyl-9-hydroxy-2-methyl-6,7,8,9-tetrahydro-4H-pyrido[1,2-a]pyrimidin-4-one 849903-79-3 C11H16N2O2 208.26 —— 3-(2-chloroethyl)-6,7,8,9,-tetrahydro-9hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one —— C10H13ClN2O2 228.678 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:PALIPERIDONE DERIVATIVES摘要:式(1)的化合物可用作药物,并用作制备帕利哌酮的中间体。其中Y是具有以下结构的酸敏感基团:其中X代表氧原子、硫原子或—NH—基团;Z代表C2-C7烷基桥;R代表氢或C1-C4烷基基团。公开号:US20090036470A1
-
作为产物:参考文献:名称:PREPARATION OF 3-[2-[4-((6-FLUORO-1, 2-BENZISOXAZOL-3-YL)-L-PIPERIDINYL)-6, 7, 8, 9-TETRAHYDRO-9-HYDROXY-2-METHYL-4H-PYRIDO[ 1, 2-A]-PYRIMIDIN-4-ONE (PALIPERIDONE) AND PALIPERIDONE PALMITATE.摘要:一种改进的合成过程,用于合成3-[2-[4-((6-氟-1,2-苯并异噁唑-3-基)-1-哌啶基]-6,7,8,9-四氢-9-羟基-2-甲基-4H-吡啶并[1,2-a]-嘧啶-4-酮(帕利哌酮)和帕利哌酮棕榈酸酯,通过一种新颖的中间体(2-氯乙基)-6,7,8,9-四氢-2-甲基-9-羟基-4H-吡啶并[1,2-a]嘧啶-4-酮棕榈酸酯。公开号:US20140073787A1
文献信息
-
药物的缩酮衍生物及其制备方法、药物组合物和用途
-
An Integrated Approach toward NanoBRET Tracers for Analysis of GPCR Ligand Engagement作者:Michael P. Killoran、Sergiy Levin、Michelle E. Boursier、Kristopher Zimmerman、Robin Hurst、Mary P. Hall、Thomas Machleidt、Thomas A. Kirkland、Rachel Friedman OhanaDOI:10.3390/molecules26102857日期:——
Gaining insight into the pharmacology of ligand engagement with G-protein coupled receptors (GPCRs) under biologically relevant conditions is vital to both drug discovery and basic research. NanoLuc-based bioluminescence resonance energy transfer (NanoBRET) monitoring competitive binding between fluorescent tracers and unmodified test compounds has emerged as a robust and sensitive method to quantify ligand engagement with specific GPCRs genetically fused to NanoLuc luciferase or the luminogenic HiBiT peptide. However, development of fluorescent tracers is often challenging and remains the principal bottleneck for this approach. One way to alleviate the burden of developing a specific tracer for each receptor is using promiscuous tracers, which is made possible by the intrinsic specificity of BRET. Here, we devised an integrated tracer discovery workflow that couples machine learning-guided in silico screening for scaffolds displaying promiscuous binding to GPCRs with a blend of synthetic strategies to rapidly generate multiple tracer candidates. Subsequently, these candidates were evaluated for binding in a NanoBRET ligand-engagement screen across a library of HiBiT-tagged GPCRs. Employing this workflow, we generated several promiscuous fluorescent tracers that can effectively engage multiple GPCRs, demonstrating the efficiency of this approach. We believe that this workflow has the potential to accelerate discovery of NanoBRET fluorescent tracers for GPCRs and other target classes.
获取有关配体在生物相关条件下与G蛋白偶联受体(GPCRs)相互作用的药理学见解对于药物发现和基础研究至关重要。基于NanoLuc的生物发光共振能量转移(NanoBRET)监测荧光示踪剂与未修改的测试化合物之间的竞争结合已经成为一种强大而敏感的方法,用于量化与特定GPCRs遗传融合到NanoLuc荧光酶或发光HiBiT肽的配体相互作用。然而,开发荧光示踪剂通常具有挑战性,并且仍然是该方法的主要瓶颈。减轻为每个受体开发特定示踪剂的负担的一种方法是使用多功能示踪剂,这是由BRET的固有特异性所实现的。在这里,我们设计了一种集成示踪剂发现工作流程,将机器学习引导的体外筛选具有对GPCRs显示多功能结合的支架与合成策略的混合相结合,以快速生成多个示踪剂候选物。随后,这些候选物在HiBiT标记的GPCRs库中进行了NanoBRET配体结合筛选的结合评估。利用这种工作流程,我们生成了几种可以有效与多个GPCRs相互作用的多功能荧光示踪剂,展示了这种方法的效率。我们相信这种工作流程有潜力加速发现用于GPCRs和其他靶标类别的NanoBRET荧光示踪剂。 -
Process for the preparation of pure 3-[2-[4-(6-fluoro-1, 2-benzisoxazol-3-yl)-1-piperidinyl]-ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido [1,2-a] pyrimidin-4-one申请人:Reddy Reddy Buchi公开号:US20060004199A1公开(公告)日:2006-01-05A process for the preparation of 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]-ethyl]-6,7,8,9-tetrahydro-2-methyl-4H-pyrido[1,2-a]pyrimidin-4-one, which is known as Risperidone of Formula (I). Risperidone of Formula (I) is represented by the following structure.
-
[EN] ACID ADDITION SALTS OF RISPERIDONE AND PHARMACEUTICAL COMPOSITIONS THEREOF<br/>[FR] SELS D'ADDITION D'ACIDE DE LA RISPÉRIDONE ET COMPOSITIONS PHARMACEUTIQUES DE CEUX-CI申请人:TORRENT PHARMACEUTICALS LTD公开号:WO2012147035A1公开(公告)日:2012-11-01The present invention relates to a novel acid addition salt of risperidone, wherein acid counterion is selected from the group consisting of pamoic acid, caproic acid, cypionic acid, decanoic acid, camphor sulfonic acid, enanthic acid, palmitic acid, fusidic acid, gluceptic acid, gluconic acid, lactobionic acid, lauric acid, levulinic acid and valeric acid, a process for the preparation and pharmaceutical composition comprising the same. Further, the invention relates to the use of said pharmaceutical composition comprising the acid addition salt of risperidone in the treatment of patient suffering from psychotic disorders.
-
Selective Chemical Oxidation of Risperidone: A Straightforward and Cost-Effective Synthesis of Paliperidone作者:Renata Riva、Luca Banfi、Graziano Castaldi、Diego Ghislieri、Luciana Malpezzi、Francesca Musumeci、Roberto Tufaro、Marcello RaspariniDOI:10.1002/ejoc.201001618日期:2011.4A very short and cost-effective synthesis of the commercial drug paliperidone has been achieved starting from its parent compound risperidone, through a thoroughly optimized oxidation with air under basic conditions.
表征谱图
-
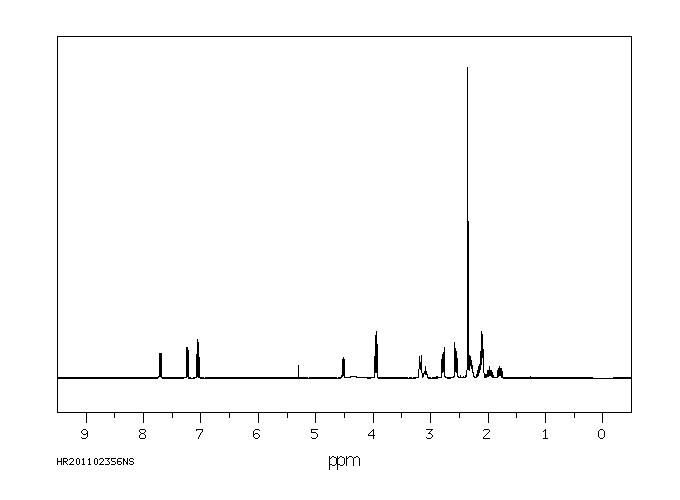
氢谱1HNMR
-
质谱MS
-
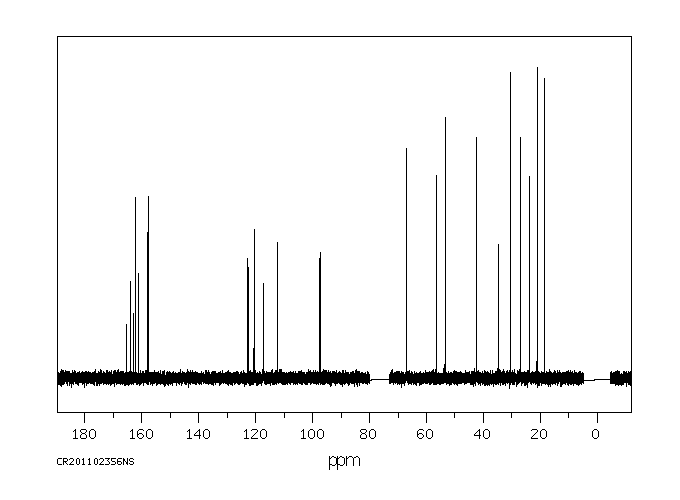
碳谱13CNMR
-
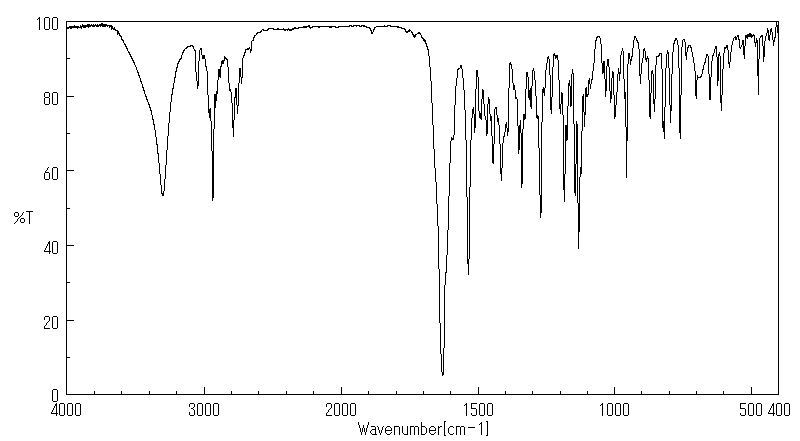
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息