1,2-二溴乙烷 | 106-93-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:9 °C
-
沸点:131-132 °C(lit.)
-
密度:2.18 g/mL at 25 °C(lit.)
-
蒸气密度:~6.5 (vs air)
-
闪点:132°C
-
溶解度:可溶于250份水中
-
暴露限值:NIOSH REL: TWA 0.045 ppm, 15-min C 0.13 ppm, IDLH 100 ppm; OSHA PEL: TWA 20 ppm, C 30 ppm, 5-min peak 50 ppm;ACGIH TLV: suspected human carcinogen.
-
介电常数:4.75
-
物理描述:Colorless liquid or solid (below 50°F) with a sweet odor.
-
颜色/状态:Heavy liquid
-
气味:Chloroform odor
-
蒸汽密度:6.48 (NTP, 1992) (Relative to Air)
-
蒸汽压力:11.2 mm Hg at 25 °C
-
亨利常数:6.50e-04 atm-m3/mole
-
大气OH速率常数:2.50e-13 cm3/molecule*sec
-
稳定性/保质期:
-
蒸气有毒,高浓度时能引起麻醉作用,全麻时会引起肺水肿致死。空气中最低中毒浓度为25×10-6,吸入致死浓度为1000×10-6。对啮齿动物有致癌作用。空气中最高容许浓度为130×10-9。蒸气刺激呼吸道并损伤肝、肾。液体接触皮肤可导致溃烂。生产场所应通风良好,并备有防毒面具和防护服等,一旦受污染需立即脱去外衣并擦干皮肤。
-
具有中度麻醉作用,易被皮肤吸收,其蒸气能刺激眼黏膜和上呼吸道。麻醉性虽小但会导致感觉迟钝、抑郁和呕吐等症状。服用40g即可致死。慢性中毒时可引发眼球结膜炎、支气管炎、喉头炎、食欲不振及抑郁等病症。嗅觉阈浓度为199.94mg/m³,工作场所最高容许浓度为192.25 mg/m³。
-
稳定性:稳定 [20]
-
禁配物:碱金属、强氧化剂 [21]
-
避免接触的条件:光照、受热 [22]
-
聚合危害:不聚合 [23]
-
分解产物:溴化氢 [24]
-
自燃温度:Not flammable (USCG, 1999)
-
分解:At 240-270 °C in a glass vessel, ethylene bromide decomposes into vinyl bromide & hydrogen bromide.
-
粘度:1.727 cP at 20 °C
-
腐蚀性:Liquid ethylene dibromide will attack some forms of plastics, rubber, and coatings.
-
燃烧热:Heat of combustion in an oxygen bomb is 6647 J/g (1289 cal/g).
-
汽化热:82.1 BTU/lb = 45.6 cal/g = 1.91X10+5 J/kg
-
表面张力:38.75 dynes/cm = 0.03875 Newtons/m at 20 °C; Liquid-water interfacial tension: 36.54 dynes at 20 °C
-
电离电位:9.45 eV
-
气味阈值:Low: 76.80 mg/cu m; High: 62.50 mg/cu m.
-
折光率:Index of refraction: 1.5356 at 20 °C/D
-
保留指数:770.9;787;795;805;786.9;775.4;787;783.2;790;774;790;823;783.9
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:4
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:E
-
职业暴露限值:TWA: 0.045 ppm, Ceiling: 0.13 ppm [15-minute]
-
TSCA:Yes
-
危险等级:6.1
-
立即威胁生命和健康浓度:46 ppm (354 mg/m3)
-
危险品标志:T
-
安全说明:S16,S26,S36/37,S36/37/39,S45,S53,S61,S7
-
危险类别码:R23/24/25,R45,R36/37/38,R51/53
-
WGK Germany:3
-
海关编码:2903310000
-
危险品运输编号:UN 1605 6.1/PG 1
-
危险类别:6.1
-
RTECS号:KH9275000
-
包装等级:I
-
储存条件:1. **储存注意事项**:应储存在阴凉、通风的库房中,远离火种和热源,并确保容器密封。需与氧化剂、碱金属及食用化学品分开存放,避免混合储存。储存区域应配备泄漏应急处理设备和合适的收容材料。 2. **贮运条件和防护要求**:同溴甲烷,注意避免与铝、镁、钾、钠等物质接触或与强碱和富氯物质接触。国际航空运输联合法规将其归类为第727条毒物B。
SDS
| 国标编号: | 61565 |
| CAS: | 106-93-4 |
| 中文名称: | 1,2-二溴乙烷 |
| 英文名称: | 1,2-dibromoethane |
| 别 名: | 乙撑二溴 |
| 分子式: | C 2 H 4 Br 2 ;BrCH 2 CH 2 Br |
| 分子量: | 187.88 |
| 熔 点: | 9.3℃ 沸点:131.4℃ |
| 密 度: | 相对密度(水=1)2.17; |
| 蒸汽压: | 30℃ |
| 溶解性: | 微溶于水,可混溶于多数有机溶剂 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色质重有甜味的液体 |
| 危险标记: | 14(有毒品) |
| 用 途: | 用作溶剂,用于有机合成,制造杀虫剂、药品等 |
2、对环境的影响
该物质对环境有危害,对大气臭氧层有极强破坏力。对哺乳动物和鸟类应给予特别注意。
一、健康危害
侵入途径:吸入、食入、经皮吸收。
健康危害:具有中度麻醉作用。对皮肤粘膜有刺激作用。重者可致肺炎和肺水肿。对中枢神经有抑制作用。可致肝、肾损害。
急性中毒:可有头痛、头晕、耳鸣、全身无力、面色苍白、恶心、呕吐,可死于心力衰竭。引起皮炎和结膜炎。
二、毒理学资料及环境行为
毒性:潜在致癌物。
急性毒性:LD 50 108mg/kg(大鼠经口);300mg/kg(兔经皮);LC 50 0.384g/m 3 (大鼠吸入);人经口140mg/kg,致死。
亚急性慢性毒性:大鼠/兔吸入0.768g/m 3 ×7小时/日×5日/周×6个月,实验动物死亡率较对照高存活动物和肺、肝、肾重量增加。
致突变性:微生物致突变:鼠伤寒沙门氏菌500nmol/皿;大肠杆菌20uL/皿。姊妹染色体交换:人淋巴细胞10nmol/L。
生殖毒性:大鼠吸入最低中毒浓度(TCL0):80ppm (24小时),致胎鼠死亡。大鼠吸入最低中毒浓度(TCL0)对睾丸、附睾、输精管、性腺、尿道及雄性生育指数有影响。
致癌性:大鼠吸入20ppm×7小时/日×18月,肝细胞癌及肝血管肉瘤等。
危险特性:受高热分解产生有毒的溴化物气体。与强氧化剂接触可发生化学反应。
燃烧(分解)产物:溴化氢。 3、现场应急监测方法
4、实验室监测方法
气相色谱法《水和废水标准检验法》19版译文,江苏省监测中心
色谱/质谱法,美国EPA524.2方法 5、环境标准
| 美国 | 车间卫生标准 | 5ppm |
| 美国(1981) | 食物和饲料中容许残留限值 | 0.001mg/kg(大豆) |
| 嗅觉阈浓度 | 26ppm |
6、应急处理处置方法
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并立即隔离150米,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿防毒服。不要直接接触泄漏物。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土、蛭石或其它惰性材料吸收。大量泄漏:构筑围堤或挖坑收容。用泡沫覆盖,降低蒸气灾害。用泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
废弃物处置方法:用控制焚烧法,焚烧系统要按装洗涤器和贮灰装
制备方法与用途
1,2-二溴乙烷是一种无色透明液体,微溶于水,能与乙醇、乙醚等多种有机溶剂混合。在光照下会稍有分解。
应用1,2-二溴乙烷可用作乙基化试剂和溶剂;在农业上用作杀线虫剂及合成植物生长调节剂;医药上用于合成二乙基溴苯乙腈中间体、溴乙烯以及乙烯叉二溴苯阻燃剂;还用作汽油抗震液中铅的消除剂、金属表面处理剂和灭火剂等。车用汽油通常采用二溴乙烷与二氯乙烷混合物以降低成本,而航空汽油则使用纯二溴乙烷。
化学性质1,2-二溴乙烷在常温常压下是一种具有挥发性的无色液体,有特殊甜味。其凝固点为9.79℃,沸点为131.4℃,在34℃(1.86kPa)时蒸气压力最大,熔点为9.9℃,冰点为-8.3℃,相对密度2.1792,折射率分别为1.5387和1.5380,粘度(20℃)为1.727mPa·s,表面张力(20℃)为38.91mN/m。常温下比较稳定,但在光照下能缓缓分解为有毒物质。它能与乙醇、乙醚、四氯化碳、苯、汽油等多种有机溶剂互溶,并形成共沸物,可溶于约250倍的水中。具有类似氯仿的气味,能乳化,并且与液氨混合至室温会发生爆炸。
用途1,2-二溴乙烷用于有机合成和作为溶剂。
用途该物质用作有机合成中间体。
生产方法1.乙烯溴化法:工业生产采用乙烯与溴进行非催化加成,反应速度随温度升高而增加。排放尾气中含过剩的乙烯和HBr,经洗涤塔用水洗涤脱除HBr后回收过剩乙烯。 2.乙烷溴化法。 3.乙二醇和溴氢酸反应法。
分类 农药 毒性分级- 高毒
- 口服毒性:大鼠 LD50: 108 毫克/公斤,小鼠 LD50: 250 毫克/公斤
- 皮肤刺激:兔子1% /14天 重度
- 眼睛刺激:兔子 1% 中度
受热分解产生溴化物气体。
储运特性库房需保持通风、低温干燥;与氧化剂和食品添加剂分开存放。
灭火剂干粉、泡沫、二氧化碳、砂土。
职业标准- 时间加权平均容许浓度(TWA):145 毫克/立方米
- 短时间接触容许浓度(STEL):225 毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 溴乙烷 ethyl bromide 74-96-4 C2H5Br 108.966 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 溴乙烷 ethyl bromide 74-96-4 C2H5Br 108.966 1,1,2-三溴乙烷 1,1,2-tribromoethane 78-74-0 C2H3Br3 266.758
反应信息
-
作为反应物:参考文献:名称:Hofmann,A. W., Chemische Berichte, 1890, vol. 23, p. 3299摘要:DOI:
-
作为产物:描述:参考文献:名称:1,2-二溴四氯乙烷:一种有效的试剂,可通过修饰的Appel反应进行许多转化摘要:已开发出一种高效且简便的方法,可在温和的条件下使用三苯基膦(PPh $ _ {3})$ / 1,2-二溴四氯乙烷(DBTCE)络合物在多种条件下从多种醇中合成烷基溴化物,且收率极高,且时间短( 5分钟)。该方法也可以用于以非常高的对映体过量将手性醇转化为其相应的溴化物。PPh $ _ {3} $ / DBTCE配合物也已成功应用于温和条件下环状醚的开环反应。通过在这项工作中对Appel反应方案进行修改,还可以在非常温和的实验条件下成功完成酯化,酰胺化和酸酐的形成。DOI:10.3906/kim-1804-41
-
作为试剂:描述:4-methyl-4-((3-phenylprop-2-yn-1-yl)oxy)cyclohexa-2,5-dien-1-one 在 diiodo(p-cymene)ruthenium(II) dimer 、 palladium diacetate 、 四丁基碘化铵 、 potassium carbonate 、 1,2-二溴乙烷 、 2-二环己基磷-2,4,6-三异丙基联苯 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 30.0h, 生成 (Z)-7a-Methyl-3-((E)-1-phenyl-3-(p-tolyl)allylidene)-2,3-dihydrobenzofuran-5(7aH)-one参考文献:名称:钌和碘阴离子共催化级联二卤化和内部炔系环己二烯与 1,2-二卤乙烷的环化摘要:我们建立了一种高效的钌(II)和碘阴离子共催化二卤化和内炔束缚环己二烯酮的级联环化,其在温和条件下以高产率立体选择性地提供了大量具有生物活性氢苯并呋喃骨架的二卤化产物。在该转化中,反应途径由亲电子碘试剂的浓度决定,这也为控制反应选择性提供了策略。此外,该方法的特点是通过碘阴离子催化剂使用1,2-二卤乙烷作为卤素源。DOI:10.1021/acs.joc.4c00951
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
Synthesis and biochemical activities of antiproliferative amino acid and phosphate derivatives of microtubule-disrupting β-lactam combretastatins作者:Niamh M. O'Boyle、Lisa M. Greene、Niall O. Keely、Shu Wang、Tadhg S. Cotter、Daniela M. Zisterer、Mary J. MeeganDOI:10.1016/j.ejmech.2013.01.016日期:2013.4The synthesis and biochemical activities of novel water-soluble β-lactam analogues of combretastatin A-4 are described. The first series of compounds investigated, β-lactam phosphate esters 7a, 8a and 9a, exhibited potent antiproliferative activity and caused microtubule disruption in human breast carcinoma-derived MCF-7 cells. They did not inhibit tubulin polymerisation in vitro, indicating that biotransformation描述了康布雷他汀A-4的新型水溶性β-内酰胺类似物的合成和生化活性。研究的第一批化合物,β-内酰胺磷酸酯7a,8a和9a,在人乳腺癌衍生的MCF-7细胞中表现出有效的抗增殖活性并引起微管破坏。它们在体外没有抑制微管蛋白的聚合,表明生物转化是其在MCF-7细胞中的抗增殖和微管蛋白结合作用所必需的。第二系列化合物,β-内酰胺氨基酸酰胺(包括10k和11l)在MCF-7细胞中显示出强大的抗增殖活性,在MCF-7细胞中破坏了微管,并且在体外也抑制了微管蛋白的聚合。这表明与磷酸盐系列相反,β-内酰胺酰胺不需要代谢活化即可具有抗增殖作用。这两个系列的化合物均引起MCF-7细胞的有丝分裂灾难和细胞凋亡。分子模型研究表明,在微管蛋白的秋水仙碱结合位点中,β-内酰胺氨基酸酰胺10k和11l具有潜在的结合构象。由于它们的水溶性和强大的生化作用,这些化合物有望作为微管破坏剂进一步开发。
-
[EN] SUBSTITUTED N-HETEROCYCLIC CARBOXAMIDES AS ACID CERAMIDASE INHIBITORS AND THEIR USE AS MEDICAMENTS<br/>[FR] CARBOXAMIDES N-HÉTÉROCYCLIQUES SUBSTITUÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA CÉRAMIDASE ACIDE ET LEUR UTILISATION EN TANT QUE MÉDICAMENTS申请人:BIAL BIOTECH INVEST INC公开号:WO2021055627A1公开(公告)日:2021-03-25The invention provides substituted N-heterocyclic carboxamides and related compounds, compositions containing such compounds, medical kits, and methods for using such compounds and compositions to treat a medical disorder, e.g., cancer, lysosomal storage disorder, neurodegenerative disorder, inflammatory disorder, in a patient.这项发明提供了替代的N-杂环羧酰胺和相关化合物,含有这些化合物的组合物,医疗工具包,以及使用这些化合物和组合物治疗患者的医疗疾病(例如癌症、溶酶体贮积症、神经退行性疾病、炎症性疾病)的方法。
-
Synthesis, characterization, catalytic and biological application of half-sandwich ruthenium complexes bearing hemilabile (κ2-<i>C</i>,<i>S</i>)-thioether-functionalised NHC ligands作者:Weiguang Chen、Julien Egly、Amalia I. Poblador-Bahamonde、Aline Maisse-Francois、Stéphane Bellemin-Laponnaz、Thierry AchardDOI:10.1039/c9dt04825a日期:——suggesting that the only species observed by the 1H-NMR correspond to an average resonance position of a fluxional mixtures of isomers. All these complexes were found to catalyse the oxydant-free double dehydrogenation of primary amine into nitrile. Ru complex bearing NHC-functionalised S-tBu group was further investigated in a wide range of amines and was found more selective for alkyl amine substrates than一系列阳离子的Ru(II)(η 6 - p -cymene)络合物与硫醚官能的N-杂环碳烯配体已经制备和完全表征。研究了R硫醚取代基对硫原子配位的立体和电子影响。他们三个的分子结构已通过X射线衍射仪来测定并证实了二齿(κ 2 - Ç,小号)配位体的配位模式。有趣的是,对于配合物1c,1i和1j,在固态下仅观察到一个单一的非对映体(对映体对)。DFT计算通过带有R供体基团的硫锥体转化途径在两个非对映异构体之间建立了一个低能转化障碍,而带有R取代基的含电子吸收基团的解离/缔合机制更可能,因此表明1 1 H-NMR对应于异构体的流动混合物的平均共振位置。发现所有这些配合物都催化伯胺的无氧化剂无双脱氢成腈。Ru复合轴承NHC功能化S- t在广泛的胺类中进一步研究了Bu基团,发现对烷基胺底物的选择性比对苄胺衍生物的选择性高。最后,报道了四种选择的Ru配合物对各种人类癌细胞的生物学效应的初步结果。
-
Acetylenic &agr;-amino acid-based sulfonamide hydroxamic acid tace inhibitors申请人:American Cyanamid Company公开号:US06225311B1公开(公告)日:2001-05-01Compounds of the formula: are useful in treating disease conditions mediated by TNF-&agr;, such as rheumatoid arthritis, osteoarthritis, sepsis, AIDS, ulcerative colitis, multiple sclerosis, Crohn's disease and degenerative cartilage loss.该式化合物在治疗由TNF-α介导的疾病条件中很有用,如类风湿性关节炎、骨关节炎、败血症、艾滋病、溃疡性结肠炎、多发性硬化症、克罗恩病和软骨退行性损失。
表征谱图
-
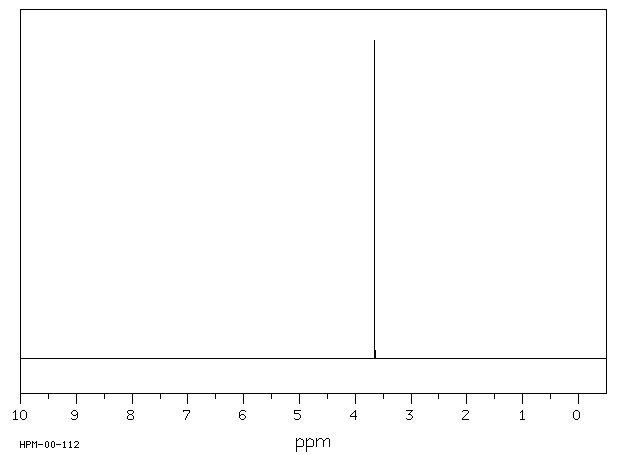
氢谱1HNMR
-
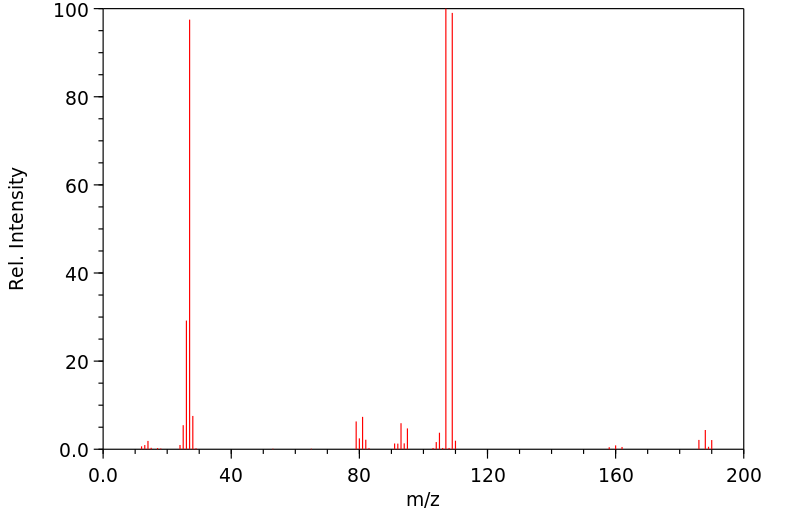
质谱MS
-
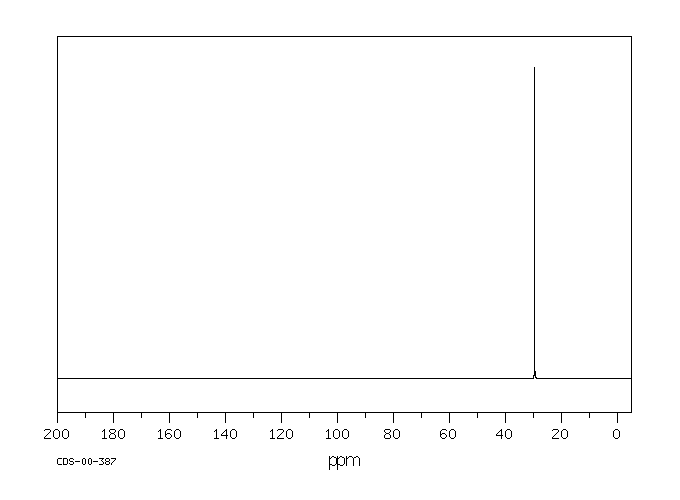
碳谱13CNMR
-
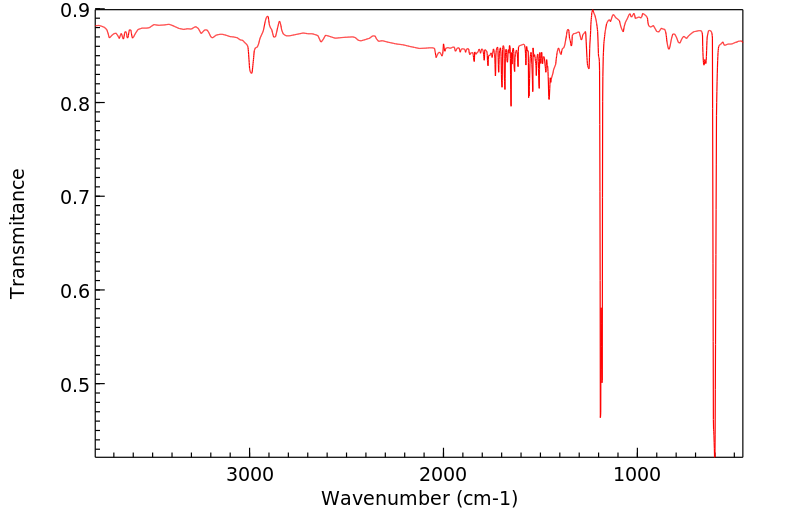
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息