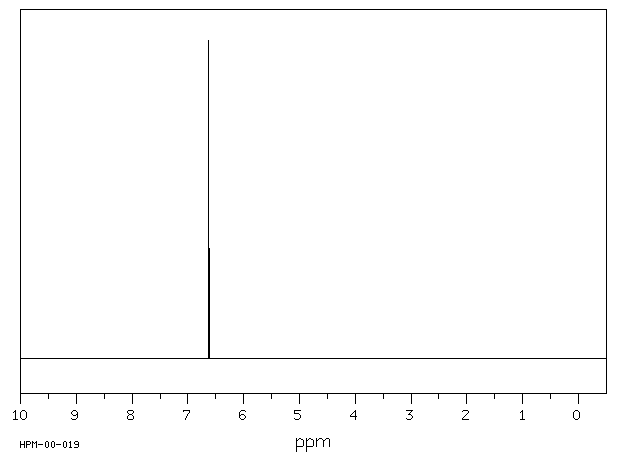
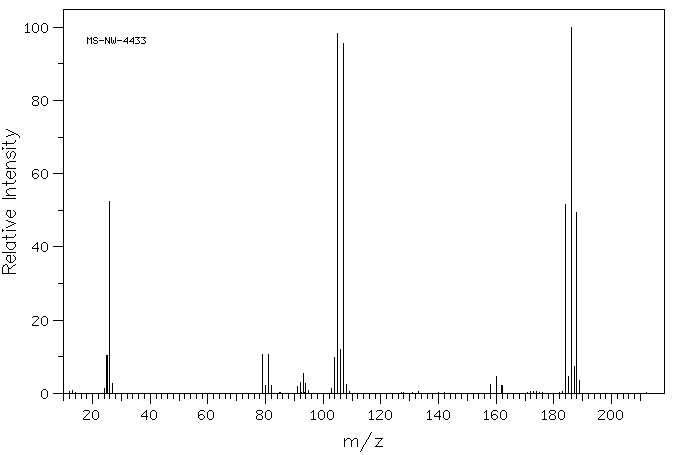
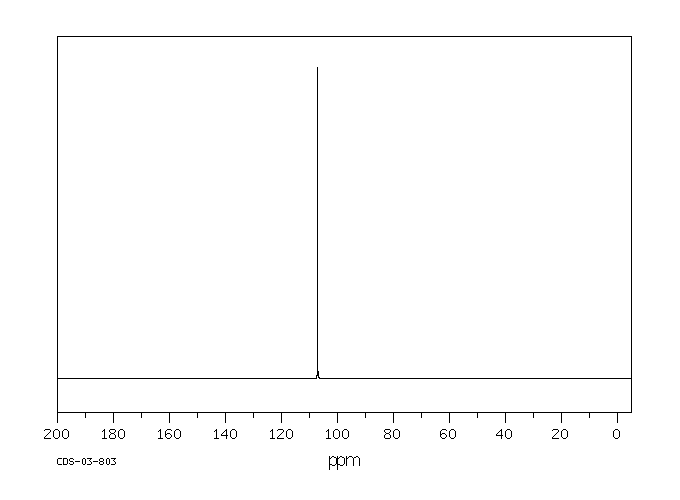
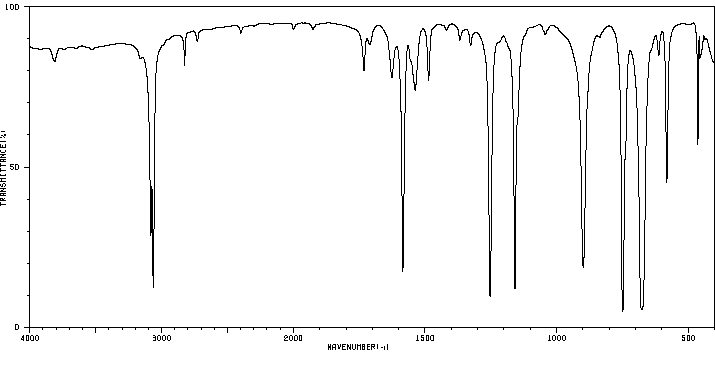
毒理性
神经毒素 - 急性溶剂综合症
皮肤毒素 - 皮肤烧伤
催泪剂(催泪瓦斯)- 刺激眼睛并引起流泪的物质
Neurotoxin - Acute solvent syndrome
Dermatotoxin - Skin burns.
Lacrimator (Lachrymator) - A substance that irritates the eyes and induces the flow of tears.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases