1,2-二环己基乙烷 | 3321-50-4
物质功能分类
中文名称
1,2-二环己基乙烷
中文别名
——
英文名称
1,2-dicyclohexylethane
英文别名
1,2-Dicyclohexyl-aethan;1,2-Dicyclohexyl-ethan;2-cyclohexylethylcyclohexane
CAS
3321-50-4
化学式
C14H26
mdl
MFCD00013768
分子量
194.36
InChiKey
IBLVSWYGUFGDMF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:11.5°C
-
沸点:270.85°C
-
密度:0.8728
-
保留指数:1498;1529;1490;1490;1507;1515
计算性质
-
辛醇/水分配系数(LogP):6.8
-
重原子数:14
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2902199090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 甲基环己烷 Methylcyclohexane 108-87-2 C7H14 98.1882
反应信息
-
作为反应物:描述:1,2-二环己基乙烷 在 platinum on activated charcoal 作用下, 生成 菲参考文献:名称:Zelinsky; Titz, Chemische Berichte, 1929, vol. 62, p. 2871摘要:DOI:
-
作为产物:描述:反式-1,2-二苯乙烯 在 乙醚 作用下, 反应 24.0h, 以49%的产率得到1,2-二环己基乙烷参考文献:名称:烷烃和二乙基醚介导的不锈钢介氢制氢及其在芳烃还原中的应用摘要:氢气可以从简单的烷烃(例如,生成Ñ戊烷,Ñ己烷等)和二乙醚(ET 2使用行星式球磨机(SUS304,弗里奇PULVERISETTE 7)O)通过机械能量,以及使用的不锈钢球和容器是产生氢气的重要因素。还使用原位产生的氢完成有机化合物的还原。尽管使用戊烷作为氢源可以促进烯烃部分的还原,但芳烃的还原可以使用Et 2 O进行。在不锈钢的成分(Fe,Cr,Ni等)中,Cr是金属因子用于从烷烃和Et 2生成氢O和Ni金属起着加氢催化剂的作用。DOI:10.1021/acs.orglett.8b00931
文献信息
-
The solvent determines the product in the hydrogenation of aromatic ketones using unligated RhCl<sub>3</sub> as catalyst precursor作者:Soumyadeep Chakrabortty、Nils Rockstroh、Stephan Bartling、Henrik Lund、Bernd H. Müller、Paul C. J. Kamer、Johannes G. de VriesDOI:10.1039/d1cy01504d日期:——Alkyl cyclohexanes were synthesized in high selectivity via a combined hydrogenation/hydrodeoxygenation of aromatic ketones using ligand-free RhCl3 as pre-catalyst in trifluoroethanol as solvent. The true catalyst consists of rhodium nanoparticles (Rh NPs), generated in situ during the reaction. A range of conjugated as well as non-conjugated aromatic ketones were directly hydrodeoxygenated to the corresponding
-
Raney Ni–Al alloy-mediated reduction of benzils in water作者:Guo-Bin Liu、Hong-Yun Zhao、Lu Dai、Thies Thiemann、Hideki Tashiro、Masashi TashiroDOI:10.3184/030823409x12506792542783日期:2009.9Raney Ni–AI alloy in a dilute aqueous alkaline solution has been shown to be a powerful reducing agent and is highly effective for the reduction of alkylbenzils and alkoxybenzils to afford the corresponding 1,2-diarylethers at 90°C, in the absence of organic solvents. 4,4′-Dinitrobenzil was transformed selectively to 1,2-bis(4-aminophenyl)-ethane.
-
Copper-Catalyzed Oxidative Homo- and Cross-Coupling of Grignard Reagents Using Diaziridinone作者:Yingguang Zhu、Tao Xiong、Wenyong Han、Yian ShiDOI:10.1021/ol5030103日期:2014.12.5Transition-metal-catalyzed cross-coupling reactions are among the most powerful synthetic transformations. This paper describes an efficient copper-catalyzed homo- and cross-coupling of Grignard reagents with di-tert-butyldiaziridinone as oxidant under mild conditions, giving the coupling products in good to excellent yields. The reaction process has a broad substrate scope and is also effective for
-
Nickel-Catalyzed Cross-Coupling of Alkyl Zinc Halides for the Formation of C(sp<sup>2</sup>)C(sp<sup>3</sup>) Bonds: Scope and Mechanism作者:Vilasâ B. Phapale、Manuel Guisán-Ceinos、Elena Buñuel、Diegoâ J. CárdenasDOI:10.1002/chem.200901913日期:2009.11.23Optimal conditions for a general Ni‐catalysed Negishi cross‐coupling of alkyl zinc halides with aryl, heteroaryl and alkenyl halides have been determined. These conditions allow the reaction to take place smoothly, with low catalyst loading, and in the presence of a wide variety of functional groups to afford products in good yields at room temperature. DFT studies on the mechanism support the occurrence
-
一种芳族化合物氢化及氢化脱氧的方法及其应用申请人:中国医学科学院药物研究所公开号:CN112851454B公开(公告)日:2022-11-04本发明属于医药技术领域,公开了一种在温和条件下芳族化合物氢化及氢化脱氧的方法以及该方法在芳族化合物及相关混合物氢化及氢化脱氧反应中的应用。具体而言,所述的方法为在合适的温度条件下使所述的芳族化合物或含有芳族化合物的混合物在溶剂中与催化剂及合适压力的氢气接触,使所述的氢、溶剂和芳族化合物在催化剂的作用下反应得到相应的氢化产物或/和脱除含氧取代基的氢化脱氧产物。本发明还公开了该方法的具体实施条件以及该方法适用的芳族化合物结构类型。本发明所用氢化及氢化脱氧反应方法反应条件温和,氢化脱氧效率高,底物适用性广泛,后处理方便,有良好的实验室和工业应用前景。
表征谱图
-
氢谱1HNMR
-
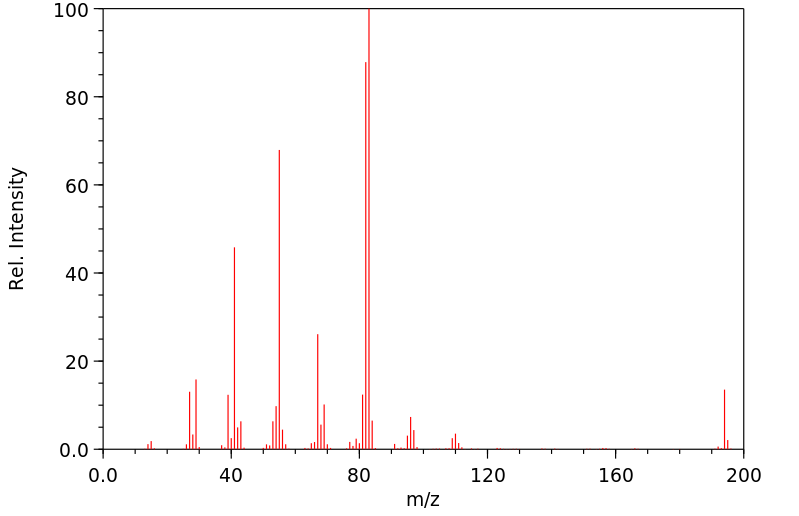
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-1-乙基-3-甲基环己烷
顺式-1-乙基-2-甲基环丙烷
顺式-1,3-二甲基环庚烷
顺式-1,2-二甲基环丙烷
顺式-1,2-二乙基环戊烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式-1,2-二(1-甲基乙基)环丙烷
顺式,反式,反式-1,2,4-三甲基环己烷
Copper, ethyl-
辛烷-d18
辛基环戊烷
辛基环丙烷
联苯肼酯
联环戊基
羰基双(环茂二烯基)钛
矿油精
癸烷,2,8-二甲基-
癸烷
decyl radical
癸基环戊烷
異十八烷
甲烷-d3
甲烷-d2
甲烷-d1
甲烷-D4
甲烷-3H
甲烷-13C,d4
甲烷-13C
甲烷
甲基自由基
甲基环辛烷
甲基环癸烷
甲基环戊烷
甲基环己烷-Me-d3
甲基环己烷
甲基环十一烷
甲基环丙烷
甲基环丁烷.
甲基丙烷-2-d
环辛烷-D16
环辛烷
环癸烷
环戊烷-D9
环戊烷-D10
环戊烷-13C1
环戊烷,三(2-辛基十二基)-
环戊烷
环戊基甲基自由基
环戊基环庚烷
环戊基环己烷