1,2-二甲基环氧乙烷 | 3266-23-7
中文名称
1,2-二甲基环氧乙烷
中文别名
2,3-环氧丁烷
英文名称
2,3-epoxybutane
英文别名
2,3-dimethyloxirane
CAS
3266-23-7
化学式
C4H8O
mdl
MFCD00066345
分子量
72.1069
InChiKey
PQXKWPLDPFFDJP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:56~57℃
-
密度:0.804 g/mL at 20 °C(lit.)
-
闪点:-15°C
-
物理描述:2,3-epoxybutane is a clear colorless liquid. (NTP, 1992)
-
熔点:-82.5 °C
-
溶解度:greater than or equal to 100 mg/mL at 68° F (NTP, 1992)
-
保留指数:511;511;512;539;539;539
-
稳定性/保质期:
在常温常压下,该物质保持稳定。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:5
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:12.5
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险品标志:F,Xi
-
安全说明:S16,S26,S36
-
危险类别码:R36/37/38,R11
-
WGK Germany:3
-
危险品运输编号:UN 3271 3/PG 2
-
RTECS号:EK3855000
-
包装等级:II
-
储存条件:常温、避光、存于通风干燥处。
SDS
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 1. 化学品
产品名称: 2,3-Butylene Oxide (cis- and trans- mixture)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3-环氧丁烷 (顺反混合物)
百分比: >95.0%(GC)
CAS编码: 3266-23-7
俗名: 2,3-Epoxybutane (cis- and trans- mixture) , Pseudobutylene Oxide (cis- and
trans- mixture)
分子式: C4H8O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: -26°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.82
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 11. 毒理学信息
生殖毒性: 无资料
RTECS 号码: EK3855000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
正式运输名称: 易燃液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 2,3-Butylene Oxide (cis- and trans- mixture)
修改号码: 6
模块 2. 危险性概述
GHS分类
物理性危害
易燃液体 第2级
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 高度易燃液体和蒸气
吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 远离热源/火花/明火/热表面。禁烟。
保持容器密闭。
使用防爆的电气/通风/照明设备。采取预防措施以防静电和火花引起的着火。
避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[储存] 存放于通风良好处。保持凉爽。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 2,3-环氧丁烷 (顺反混合物)
百分比: >95.0%(GC)
CAS编码: 3266-23-7
俗名: 2,3-Epoxybutane (cis- and trans- mixture) , Pseudobutylene Oxide (cis- and
trans- mixture)
分子式: C4H8O
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,二氧化碳
不适用的灭火剂: 水(有可能扩大灾情。)
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:喷水,保持容器冷却。如果安全,消除一切火源。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 回收到密闭容器前用干砂或惰性吸收剂吸收泄漏物。一旦大量泄漏,筑堤控制。附着
物或收集物应该根据相关法律法规废弃处置。
副危险性的防护措施 移除所有火源。一旦发生火灾应该准备灭火器。使用防火花工具和防爆设备。
模块 7. 操作处置与储存
处理
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 7. 操作处置与储存
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。远离热源/火花/明火
/热表面。禁烟。采取措施防止静电积累。使用防爆设备。处理后彻底清洗双手和脸。
注意事项: 如果可能,使用封闭系统。如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗、通风良好处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 无色-几乎无色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: -26°C
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 0.82
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
避免接触的条件: 火花, 明火, 静电
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
2,3-环氧丁烷 (顺反混合物) 修改号码:6
模块 11. 毒理学信息
生殖毒性: 无资料
RTECS 号码: EK3855000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧,焚烧时需要特别注
意该物质是高度可燃的。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第3类 易燃液体 。
UN编号: 1993
正式运输名称: 易燃液体, 不另作详细说明
包装等级: II
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 仲丁醇 iso-butanol 78-92-2 C4H10O 74.1228
反应信息
-
作为反应物:描述:1,2-二甲基环氧乙烷 在 Ipc2BBr 作用下, 以 正戊烷 为溶剂, 反应 0.5h, 以69%的产率得到3-bromo-2-butanol参考文献:名称:内消旋环氧化物与 B-卤代二异松蒎基硼烷的对映选择性环裂解摘要:摘要:B-卤代二异松蒎基硼烷,IpcBX,特别是溴化物 1b 和碘化物 1c,可区分内消旋环氧化物的对映体碳氧键,以提供中等至优异对映体纯度的 1,2-卤代醇。因此 (-)-(1R, 2R)-2-溴环己醇,(-)-(1R, 2R)-2-碘代环己醇,(-)-(1R, 2R)-2-碘代环己-4-en-1-o1和 (-)-(1R, 2R)-2-bromocyclohex-4-en-1-o1 分别以 84%、91%、63% 和 95% ee 从相应的内消旋环氧化物和卤代硼烷试剂中获得。然后从戊烷中简单重结晶得到基本上 100% ee 的卤代醇;顺式-2-氧化丁烯和顺式-3-氧化己烯分别以78% 和69% ee 提供相应的(1R, 2R)-碘醇。在所有情况下,内消旋环氧化物的 S 碳都被选择性裂解。这是对内消旋环氧化物进行对映选择性开环以获得光学活性 1,2-卤代醇的第一个例子。关键词:不对称合成。DOI:10.1021/ja00226a050
-
作为产物:描述:参考文献:名称:Ramacciotti, Alessio; Fiaschi, Rita; Napolitano, Elio, Gazzetta Chimica Italiana, 1995, vol. 125, # 3, p. 111 - 114摘要:DOI:
-
作为试剂:描述:参考文献:名称:Synthesis of 9,10-Phenanthrenequinones by Photocyclization of Derivatives of Benzoins: Scope and Limitation of the Methodology摘要:Symmetric 9,10-phenanthrenequinones with methyl, methoxy, and chloro substituents at the 3 and 6 positions have been synthesized by photocyclization of 4,5-bis-(aryl)-2-phenyl-1,3,2-dioxaboroles and 2,3-diaryldioxenes followed by oxidative hydrolysis.DOI:10.1080/00397919808003073
文献信息
-
[EN] TRIAZOLE AGONISTS OF THE APJ RECEPTOR<br/>[FR] TRIAZOLES AGONISTES DU RÉCEPTEUR APJ申请人:AMGEN INC公开号:WO2016187308A1公开(公告)日:2016-11-24Compounds of Formula I and Formula II, pharmaceutically acceptable salt thereof, stereoisomers of any of the foregoing, or mixtures thereof are agonists of the APJ Receptor and have use in treating cardiovascular and other conditions. Compounds of Formula I and Formula II have the following structures where the definitions of the variables are provided herein.公式I和公式II的化合物,其药用盐,上述任何一种的立体异构体,或它们的混合物是APJ受体的激动剂,并可用于治疗心血管和其他疾病。公式I和公式II的化合物具有以下结构,其中变量的定义在此提供。
-
Silylation reactions on nanoporous gold <i>via</i> homolytic Si–H activation of silanes作者:Hongbo Li、Huifang Guo、Zhiwen Li、Cai Wu、Jing Li、Chunliang Zhao、Shuangxi Guo、Yi Ding、Wei He、Yadong LiDOI:10.1039/c8sc01427b日期:——hydrogenation reactions. Herein we discovered homolytic activation of Si–H bonds on the surface of nanoporous gold (NPG), forming hydrogen radicals and [Au]–[Si] intermediates. By virtue of this new reactivity, we achieved highly selective mono and sequential alcoholysis of dihydrosilane. In addition, the amphiphilic nature of the [Au]–[Si] intermediate allows for a new bis-silylation reaction of cyclic ethers
-
Preparation of Di- and polynitrates by ring-opening nitration of epoxides by dinitrogen pentoxide (N2O5)作者:Peter Golding、Ross W Millar、Norman C Paul、David H RichardsDOI:10.1016/s0040-4020(01)87978-3日期:1993.8hydrocarbon solvents (principally CH2Cl2) to give vicinal nitrate ester products by a novel ring-opening nitration reaction. The procedure offers easier temperature control and simpler isolation procedures compared with conventional mixed acid nitrations; it also enables selective nitration reactions to be carried out on polyfunctional substrates. The scope and limitations of the reaction, as well as
-
Tetranitromethane as an efficient reagent for the conversion of epoxides into β-hydroxy nitrates作者:Yuliya A. Volkova、Olga A. Ivanova、Ekaterina M. Budynina、Elena B. Averina、Tamara S. Kuznetsova、Nikolai S. ZefirovDOI:10.1016/j.tetlet.2008.04.050日期:2008.6A convenient regioselective method for the preparation of β-hydroxy nitrates based on the ring opening reaction of epoxides by tetranitromethane in the presence of triethylamine is described. A series of substituted β-hydroxy nitrates were obtained in high yields under mild conditions.
-
[EN] 5-ALKYLTHIO-7-[(4-ARYLBENZYL)AMINO]-1(2)H-PYRAZOLO[4,3-D]PYRIMIDINES FOR TREATMENT OF LYMPHOMA<br/>[FR] 5-ALKYLTHIO-7-[(4-ARYLBENZYL) AMINO] -1 (2) H-PYRAZOLO [4,3-D] PYRIMIDINES POUR LE TRAITEMENT DU LYMPHOME申请人:UNIV PALACKEHO公开号:WO2019149295A1公开(公告)日:2019-08-08The present invention relates to 5-alkylthio-7-[(4-arylbenzyl)amino]-1(2)H-pyrazolo[4,3-d]pyrimidine derivatives of formula I which are effective inhibitors of kinases and exhibit strong antiproliferative and proapoptotic properties on lymphoma cells. This invention further relates to use of said derivatives in the treatment of blood hyperproliferative diseases, such as Non-Hodgkin lymphomas.
表征谱图
-
氢谱1HNMR
-
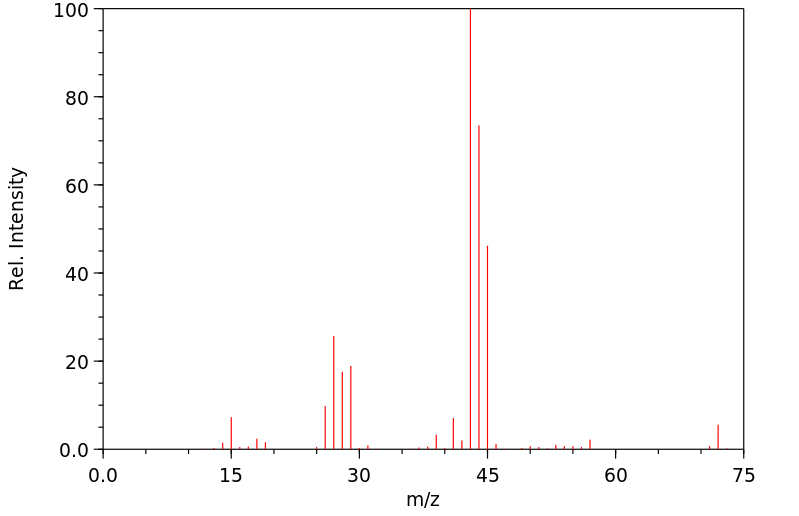
质谱MS
-
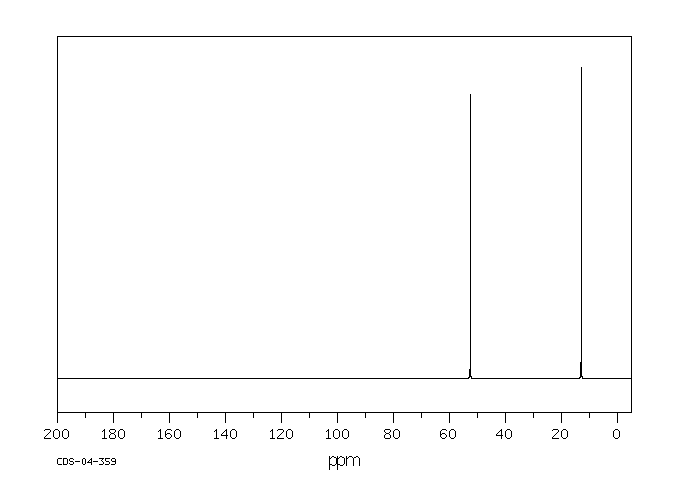
碳谱13CNMR
-
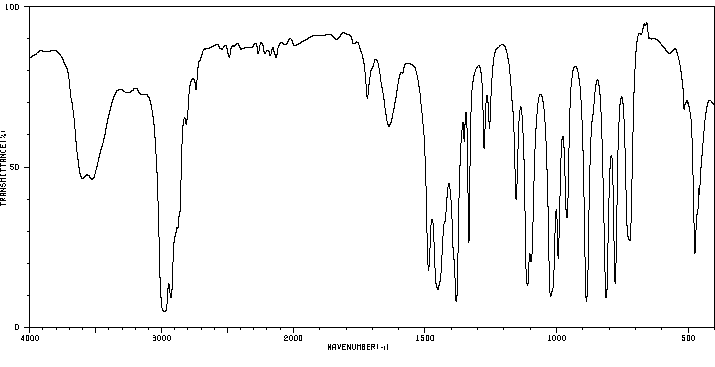
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-氯-1,2-环氧丁烷
顺式-环氧琥珀酸氢钾
顺式-1-环己基-2-乙烯基环氧乙烷
顺-(2S,3S)甲基环氧肉桂酸酯
雌舞毒蛾引诱剂
阿洛司他丁
辛基缩水甘油醚
试剂(3S,6S)-(-)-3,6-Diisopropyl-1,4-dioxane-2,5-dione
表氰醇
螺[环氧乙烷-2,2-三环[3.3.1.1~3,7~]癸烷]
蛇根混合碱
benzene oxide
聚碳酸丙烯酯
聚依他丁
羟基乙醛
缩水甘油基异丁基醚
缩水甘油基十六烷基醚
缩水甘油
硬脂基醇聚氧乙烯聚氧丙烯醚
硅烷,三甲基[(3-甲基噁丙环基)乙炔基]-,顺-
盐酸司维拉姆
甲醛与(氯甲基)环氧乙烷,4,4-(1-甲基乙亚基)双酚和2-甲基苯酚的聚合物
甲醛与(氯甲基)环氧乙烷,4,4'-(1-甲基乙亚基)二[苯酚]和4-(1,1,3,3-四甲基丁基)苯酚的聚合物
甲醇环氧乙烷与壬基酚的聚合物
甲胺聚合物与(氯甲基)环氧乙烷
甲硫代环氧丙烷
甲基环氧氯丙烷
甲基环氧巴豆酸酯
甲基环氧乙烷与环氧乙烷和十六烷基或十八烷基醚的聚合物
甲基环氧乙烷与[(2-丙烯基氧基)甲基]环氧乙烷聚合物
甲基环氧丙醇
甲基环氧丙烷
甲基N-丁-3-烯酰甘氨酸酸酯
甲基7-氧杂双环[4.1.0]庚-2,4-二烯-1-羧酸酯
甲基3-环丙基-2-环氧乙烷羧酸酯
甲基1-氧杂螺[2.5]辛烷-2-羧酸酯
甲基(2S,3R)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3S)-3-丙基-2-环氧乙烷羧酸酯
甲基(2R,3R)-3-环丙基-2-环氧乙烷羧酸酯
环氧溴丙烷
环氧氯丙烷与双酚A、4-(1,1-二甲乙基)苯酚的聚合物
环氧氯丙烷-d5
环氧氯丙烷-D1
环氧氯丙烷-3,3’-亚氨基二丙胺的聚合物
环氧氯丙烷-2-13C
环氧氯丙烷
环氧氟丙烷
环氧树脂(环氧氯丙烷和二乙二醇)
环氧树脂
环氧柏木烷