1,4-二碘代丁烷 | 628-21-7
中文名称
1,4-二碘代丁烷
中文别名
四亚甲基二碘;1,4-二碘丁烷
英文名称
1,4-Diiodobutane
英文别名
diiodobutane
CAS
628-21-7
化学式
C4H8I2
mdl
MFCD00001099
分子量
309.917
InChiKey
ROUYUBHVBIKMQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:6 °C (lit.)
-
沸点:147-152 °C/26 mmHg (lit.)
-
密度:2.35 g/mL at 25 °C (lit.)
-
闪点:108-110°C/10mm
-
稳定性/保质期:
请远离氧化剂、还原剂、光线和碱性环境。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:6
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险等级:6.1(b)
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29033080
-
危险品运输编号:2810
-
RTECS号:EJ9200000
-
包装等级:III
-
危险类别:6.1(b)
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。请将储存地点远离氧化剂,并避免阳光直射。
SDS
1,4-二碘丁烷(含稳定剂铜屑)
模块 1. 化学品
产品名称: 1,4-Diiodobutane (stabilized with Copper chip)
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,4-二碘丁烷(含稳定剂铜屑)
百分比: >98.0%(GC)
CAS编码: 628-21-7
俗名: Tetramethylene Iodide (stabilized with Copper chip)
1,4-二碘丁烷(含稳定剂铜屑)
模块 3. 成分/组成信息
分子式: C4H8I2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
加入少量的铜屑作稳定剂。必要时进行过滤分离。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
光敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
1,4-二碘丁烷(含稳定剂铜屑)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-红黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 125 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 2.36
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: EJ9200000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。废弃处置时遵守国家、地区和当地的所有法规。
1,4-二碘丁烷(含稳定剂铜屑)
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1,4-Diiodobutane (stabilized with Copper chip)
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1,4-二碘丁烷(含稳定剂铜屑)
百分比: >98.0%(GC)
CAS编码: 628-21-7
俗名: Tetramethylene Iodide (stabilized with Copper chip)
1,4-二碘丁烷(含稳定剂铜屑)
模块 3. 成分/组成信息
分子式: C4H8I2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
加入少量的铜屑作稳定剂。必要时进行过滤分离。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放于惰性气体环境中。
远离不相容的材料比如氧化剂存放。
光敏, 气敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
1,4-二碘丁烷(含稳定剂铜屑)
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 极淡的黄色-红黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 125 °C/1.6kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 2.36
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 碘化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: EJ9200000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。废弃处置时遵守国家、地区和当地的所有法规。
1,4-二碘丁烷(含稳定剂铜屑)
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:Grischkewitsch-Trochimowski, Zhurnal Russkago Fiziko-Khimicheskago Obshchestva, 1916, vol. 48, p. 913摘要:DOI:
-
作为产物:参考文献:名称:二碘四磷(P 2 I 4)是一种从醇类区域选择性合成碘代烷烃的有价值的试剂摘要:P 2 I 4在CS 2中和20°C时,伯,仲和叔醇区域选择性地转化,并以高收率的烷基碘化物转化。DOI:10.1016/s0040-4039(01)86222-5
-
作为试剂:描述:叔丁基(4-碘丁氧基)二甲基硅烷 、 (2S,4S,4aS,5aR,10aS,11aR)-4-Benzyloxy-2-benzyloxymethyl-5a-methyl-decahydro-1,5,10-trioxa-cyclohepta[b]naphthalene-9-thione 在 1,2,2,6,6-五甲基哌啶 、 1,4-二碘代丁烷 、 叔丁基锂 、 lithium,azanidylidenemethylidenecopper,2H-thiophen-2-ide 作用下, 生成参考文献:名称:Nicolaou; Reddy, K. Raja; Skokotas, Golfo, Journal of the American Chemical Society, 1993, vol. 115, # 9, p. 3558 - 3575摘要:DOI:
文献信息
-
Total Synthesis of (−)-Sarain A作者:Michael H. Becker、Peter Chua、Robert Downham、Christopher J. Douglas、Neil K. Garg、Sheldon Hiebert、Stefan Jaroch、Richard T. Matsuoka、Joy A. Middleton、Fay W. Ng、Larry E. OvermanDOI:10.1021/ja074300t日期:2007.10.1studies toward the complex marine alkaloid sarain A. Various strategies were conceived, setbacks encountered, and solutions developed, ultimately leading to a successful enantioselective total synthesis. Our route to (+)-sarain A features a number of key steps, including an asymmetric Michael addition to install the C4'-C3'-C7' stereotriad, an enoxysilane-N-sulfonyliminium ion cyclization to set the C3 quaternary
-
Copper bis(oxazolines) as catalysts for stereoselective aziridination of styrenes with N-tosyloxycarbamates作者:Hélène Lebel、Michaël Parmentier、Olivier Leogane、Karen Ross、Cédric SpitzDOI:10.1016/j.tet.2012.02.044日期:2012.4styrenes. Subsequent ring-opening reactions of styrene-derived aziridines at the benzylic position were observed with various oxygen and nitrogen nucleophiles under Lewis acid catalysis affording the corresponding products with complete inversion of stereochemistry. The strategy was used to synthesize the β-blocker, (R)-nifenalol.
-
Diphenylphosphinoyl-mediated synthesis of ketones作者:David J. Fox、Daniel Sejer Pedersen、Stuart WarrenDOI:10.1039/b606873a日期:——α-Diphenylphosphinoyl ketones are selectively and sequentially alkylated at the α-position. Double lithiation and selective alkylation occurs at the less stabilised γ-position. Dephosphinoylation of the alkylation products gives ketones. Mono-alkylation is selective, highly crystalline intermediates are formed and a one-pot strategy is possible. The method is ideally suited for the preparation of acid-sensitive ketones.
-
Nucleophilic 5-endo-trig cyclization of 2-(trifluoromethyl)allylic metal enolates and enamides: Synthesis of tetrahydrofurans and pyrrolidines bearing exo-difluoromethylene units作者:Takeshi Fujita、Masahiro Hattori、Masaaki Matsuda、Ryutaro Morioka、Tanner C. Jankins、Masahiro Ikeda、Junji IchikawaDOI:10.1016/j.tet.2018.11.011日期:2019.1proceeded exclusively in each case to afford the corresponding five-membered heterocycles with both exo-difluoromethylene and exo-alkylidene units. On treatment with potassium hexamethyldisilazide (KHMDS) or lithium diisopropylamide (LDA), 2-(trifluoromethyl)allylic ketones or imines provided the corresponding tetrahydrofurans or pyrrolidines bearing a Z-alkylidene group with perfect or substantial stereoselectivity
-
Site-Selective C–H alkylation of Complex Arenes by a Two-Step Aryl Thianthrenation-Reductive Alkylation Sequence作者:Beatrice Lansbergen、Paola Granatino、Tobias RitterDOI:10.1021/jacs.1c03459日期:2021.6.2undirected para-selective two-step C–H alkylation of complex arenes useful for late-stage functionalization. The combination of a site-selective C–H thianthrenation with palladium-catalyzed reductive electrophile cross-coupling grants access to a diverse range of synthetically useful alkylated arenes which cannot be accessed otherwise with comparable selectivity, diversity, and practicality. The robustness
表征谱图
-
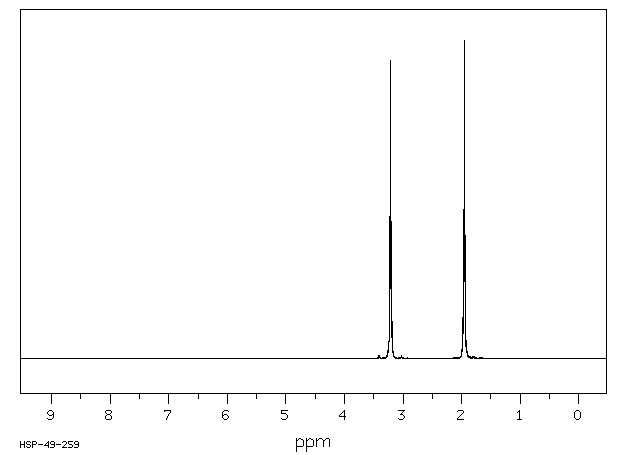
氢谱1HNMR
-
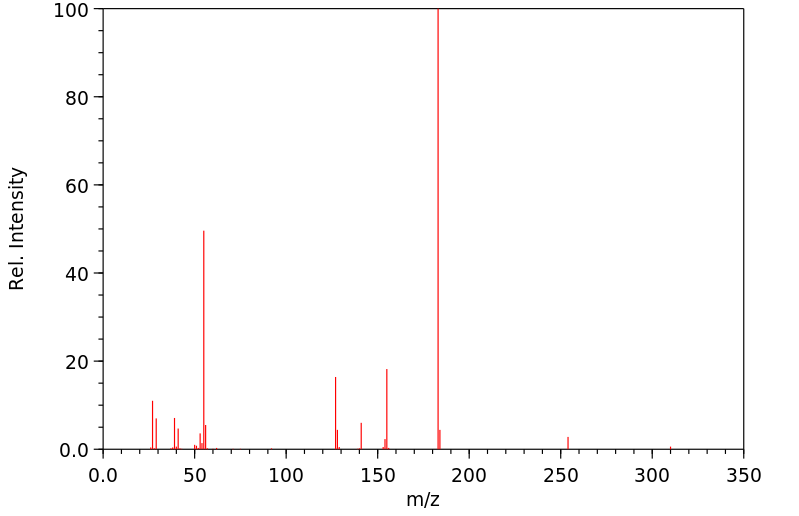
质谱MS
-
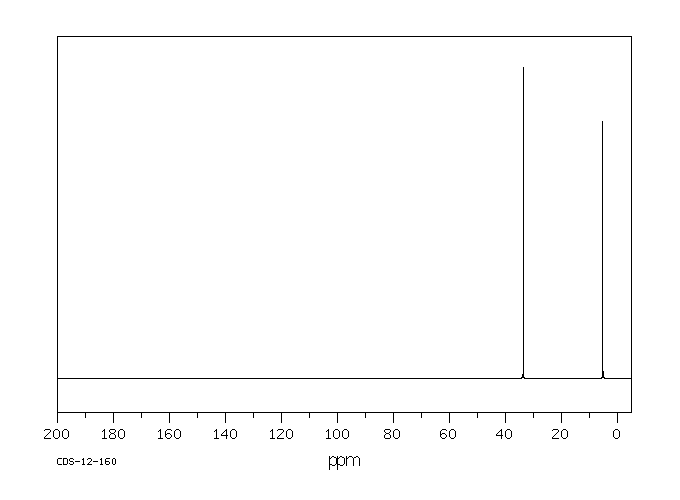
碳谱13CNMR
-
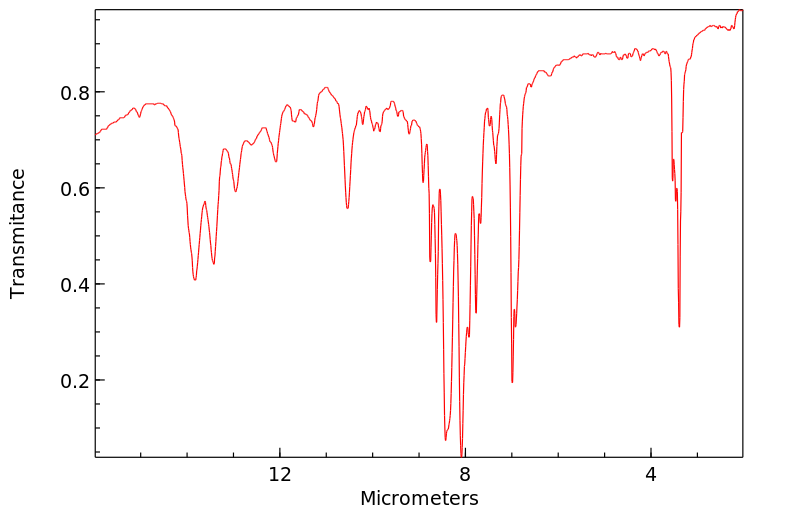
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物