代谢
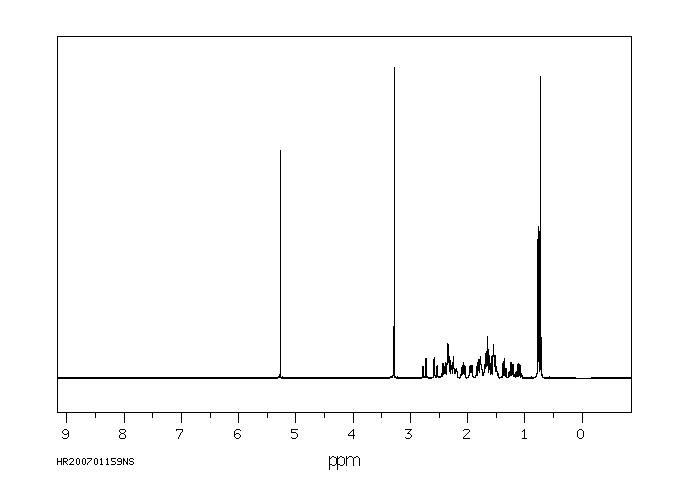
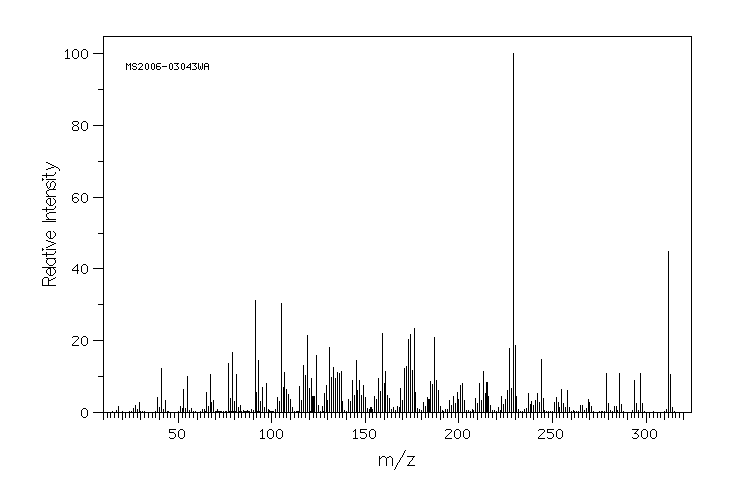
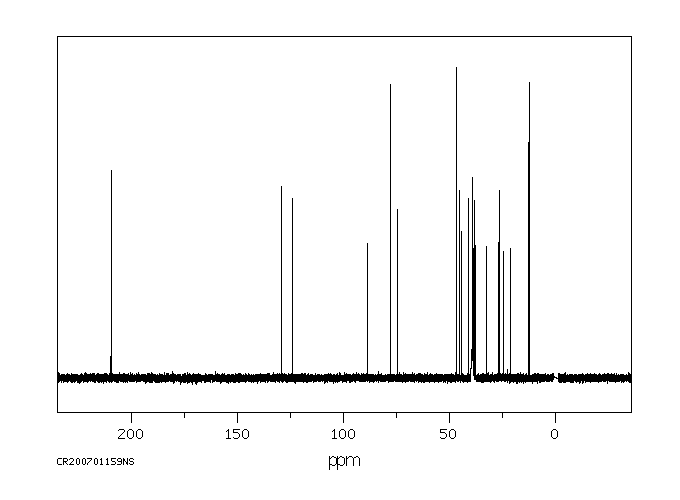
Tibolone 主要在肝脏中代谢。细胞色素P450同工酶系统参与Tibolone的轻度羟基化。Tibolone迅速转化为三种主要代谢物:3α-和3β-羟基-Tibolone具有雌激素作用,以及Δ(4)-异构体,具有孕激素和雄激素作用。3-羟基代谢物在循环中存在,主要是它们的无效硫酸化形式。
Tibolone is metabolized mainly in the liver. The cytochrome P450 isoenzyme system is involved in minor hydroxylation of tibolone. Tibolone is rapidly converted into three major metabolites: 3 alpha- and 3 beta-hydroxy-tibolone, which have oestrogenic effects, and the Delta(4)-isomer, which has both progestogenic and androgenic effects. The 3-hydroxy metabolites are present in the circulation, predominantly in their inactive sulfated form.
来源:DrugBank