1-乙炔基环戊醇 | 17356-19-3
中文名称
1-乙炔基环戊醇
中文别名
1-乙炔环戊醇;1-乙炔基-1-环戊醇;乙烯基环戊醇
英文名称
1-ethynylcyclopentanol
英文别名
1-ethynylcyclopentan-1-ol
CAS
17356-19-3
化学式
C7H10O
mdl
MFCD00001365
分子量
110.156
InChiKey
LQMDOONLLAJAPZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:27 °C
-
沸点:156-159 °C(lit.)
-
密度:0.962 g/mL at 25 °C(lit.)
-
闪点:120 °F
-
稳定性/保质期:
在常温常压下稳定,应避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.2
-
重原子数:8
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.714
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xn
-
安全说明:S16,S26,S27,S36/37/39
-
危险类别码:R22,R10,R36/37
-
WGK Germany:3
-
海关编码:2906199090
-
危险品运输编号:UN 1986 3/PG 3
-
包装等级:III
-
危险类别:3
-
危险标志:GHS02,GHS07
-
危险性描述:H226,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:常温下请将产品置于密闭容器中,避免阳光直射,并保持环境干燥和通风。
SDS
| Name: | 1-Ethynylcyclopentanol 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 17356-19-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 17356-19-3 | 1-Ethynylcyclopentanol, 98% | 98% | 241-385-7 |
Risk Phrases: 10 22 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Harmful if swallowed. Irritating to eyes, respiratory system and skin.Harmful.Flammable liquid.
Potential Health Effects
Eye:
Causes eye irritation. Lachrymator (substance which increases the flow of tears). Lachrymator (substance which increases the flow of tears).
Skin:
Causes skin irritation.
Ingestion:
Harmful if swallowed. May cause severe gastrointestinal tract irritation with nausea, vomiting and possible burns.
Inhalation:
Causes respiratory tract irritation. May cause abdominal pain, nausea, vomiting, and inflammation of the gums and mouth. May cause chemical bronchitis with coughing and difficulty in breathing.
Chronic:
Not available.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container.
Section 7 - HANDLING and STORAGE
Handling:
Use spark-proof tools and explosion proof equipment. Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a cool, dry place. Store in a tightly closed container. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower.
Exposure Limits CAS# 17356-19-3: Personal Protective Equipment Eyes: Wear chemical splash goggles.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 156 - 159 deg C @ 760.00mm Hg
Freezing/Melting Point: 27 deg C
Autoignition Temperature: Not available.
Flash Point: 48 deg C ( 118.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9620g/cm3
Molecular Formula: C7H10O
Molecular Weight: 110.16
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Not available.
Incompatibilities with Other Materials:
Strong acids - strong oxidizing agents - acid chlorides - strong reducing agents - acid anhydrides.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 17356-19-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Ethynylcyclopentanol, 98% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ALCOHOLS, FLAMMABLE, TOXIC, N.O.S.*
Hazard Class: 3 (6.1)
UN Number: 1986
Packing Group: III
IMO
Shipping Name: ALCOHOLS, TOXIC, N.O.S.
Hazard Class: 3.3 (6.1)
UN Number: 1986
Packing Group: III
RID/ADR
Shipping Name: ALCOHOLS, FLAMMABLE, TOXIC, N.O.S.
Hazard Class: 3
UN Number: 1986
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 10 Flammable.
R 22 Harmful if swallowed.
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 16 Keep away from sources of ignition - No
smoking.
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 17356-19-3: No information available.
Canada
CAS# 17356-19-3 is listed on Canada's NDSL List.
CAS# 17356-19-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 17356-19-3 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(bromoethynyl)cyclopentan-1-ol —— C7H9BrO 189.052 —— 1-(iodoethynyl)cyclopentanol 147727-80-8 C7H9IO 236.052 —— 1-Vinylaethinyl-cyclopentan-1-ol 2696-23-3 C9H12O 136.194 1-[2-(1-羟基环戊基)乙炔基]环戊烷-1-醇 1,1'-(ethyne-1,2-diyl)bis(cyclopentan-1-ol) 5325-62-2 C12H18O2 194.274 1,4-双(1-羟基环戊基)-1,3-丁二炔 1,1'-(buta-1,3-diyne-1,4-diyl)bis(cyclopentan-1-ol) 7179-09-1 C14H18O2 218.296 1-乙氧基-1-乙炔基环戊烷 1-ethoxy-1-ethynylcyclopentane 147298-23-5 C9H14O 138.21 —— chloromethyl 1-ethynylcyclopent-1-yl ether 117983-66-1 C8H11ClO 158.628 —— 1-<1-Hydroxy-cyclohexyl>-2-<1-hydroxy-cyclopentyl>-acetylen 70430-95-4 C13H20O2 208.301 1-(烯丙氧基)-1-乙炔基环戊烷 1-(allylloxy)-1-ethynylcyclopentane 121983-06-0 C10H14O 150.221 1-乙基环戊醇 1-ethylcyclopentanol 1462-96-0 C7H14O 114.188
反应信息
-
作为反应物:描述:参考文献:名称:咪唑并[1,2-a]吡啶和咪唑并[2,1-b]噻唑与含叔羟基取代基的环烷基或饱和杂环的合成摘要:已开发出一种新方法,用于合成与环烷基或饱和烷基相连的咪唑并[1,2- a ]吡啶,咪唑并[2,1– b ]噻唑和苯并[ d ]咪唑并[2,1– b ]噻唑含叔羟基取代的杂环。用溴羟基环烷基乙酮处理易得的取代的2-氨基吡啶,2-氨基噻唑和2-氨基苯并噻唑,以高收率提供所需的产品。DOI:10.1002/jhet.3454
-
作为产物:描述:参考文献:名称:摘要:Effects of structural factors in silyl ethers derived from terminal acetylenic alcohols on 1,4-O-->C-sp migration of the silyl group in the Iotsitch reagent were studied. The effect of steric factor at the carbon atom neighboring to the reaction center was found to be stronger than that at the silicon atom in the migrating group.DOI:10.1023/a:1025529428307
-
作为试剂:参考文献:名称:使用 SF6 进行光氧化还原催化 α-甲基-和 α-苯基苯乙烯的 α-烷氧基五氟磺酰化反应。摘要:采用SF6作为五氟硫酰化试剂,通过光氧化还原催化一步法制备带有邻位SF5取代基的醚。该方法显示了适用于 α-甲基和 α-苯基苯乙烯转化的醇的广泛底物范围。该产品具有一种新的结构主题,一步安装了两个功能组。烷氧基可以消除和叠氮化,进一步转化为有价值的五氟磺酰化化合物。这些结果证实,如果通过光氧化还原催化适当激活,无毒的 SF6 是一种有用的 SF5 转移试剂,并且完全避免有毒试剂。与光作为能源相结合,实现了高水平的可持续性。通过这种方法,未来可能会开发SF5取代基在药物化学、农业化学和材料化学中的潜在潜力。DOI:10.1002/anie.201910830
文献信息
-
Substrate Activity Screening: A Fragment-Based Method for the Rapid Identification of Nonpeptidic Protease Inhibitors作者:Warren J. L. Wood、Andrew W. Patterson、Hiroyuki Tsuruoka、Rishi K. Jain、Jonathan A. EllmanDOI:10.1021/ja0547230日期:2005.11.1A new fragment-based method for the rapid development of novel and distinct classes of nonpeptidic protease inhibitors, Substrate Activity Screening (SAS), is described. This method consists of three steps: (1) a library of N-acyl aminocoumarins with diverse, low molecular weight N-acyl groups is screened to identify protease substrates using a simple fluorescence-based assay, (2) the identified N-acyl描述了一种新的基于片段的方法,用于快速开发新型和不同类别的非肽蛋白酶抑制剂,即底物活性筛选 (SAS)。该方法由三个步骤组成:(1) 筛选具有多种低分子量 N-酰基的 N-酰基氨基香豆素库,以使用简单的基于荧光的测定来鉴定蛋白酶底物,(2) 已鉴定的 N-酰基氨基香豆素底物通过快速类似物合成和评估进行优化,并且 (3) 优化的底物通过用已知机制的药效团直接替换氨基香豆素而转化为抑制剂。SAS 方法已成功应用于与自身免疫性疾病有关的半胱氨酸蛋白酶组织蛋白酶 S。在筛选 N-酰基氨基香豆素文库时鉴定出多种不同类别的非肽底物。两种非肽底物类别针对底物进行了优化,每个类别的切割效率提高了 8000 倍以上。然后将选择的非肽底物直接转化为对组织蛋白酶 S 具有纳摩尔亲和力的低分子量新型醛抑制剂。本研究证明了这种基于底物的快速鉴定和优化弱片段方法的独特特征和优点,并提供了框架用于开发针对许多不同蛋白酶的完全非肽抑制剂。
-
Synthesis and click reaction of tubulin polymerization inhibitor 9-azido-α-noscapine作者:Naresh Kumar Manchukonda、Praveen Kumar Reddy Nagireddy、Balasubramanian Sridhar、Srinivas KantevariDOI:10.1007/s11164-016-2773-7日期:2017.4Abstract An efficient protocol for the synthesis of tubulin polymerization inhibitor, 9-azido-α-noscapine 2h from 9-amino-α-noscapine 2g is developed using mild reaction conditions ( t -butyl nitrite/trimethylsilyl azide in acetonitrile at room temperature). Operational simplicity, high product yield without formation of any side products are the advantages of this protocol. Further copper catalyzed
-
NOVEL DIHYDROPYRIMIDINOISOQUINOLINONES AND PHARMACEUTICAL COMPOSITIONS THEREOF FOR THE TREATMENT OF INFLAMMATORY DISORDERS.申请人:GALAPAGOS NV公开号:US20130165437A1公开(公告)日:2013-06-27A compound according to Formula Ia: wherein L 1 , G, and R 1 are as described herein. The present invention relates to novel compounds according to Formula I that antagonize GPR84, a G-protein-coupled receptor that is involved in inflammatory conditions, and methods for the production of these novel compounds, pharmaceutical compositions comprising these compounds, and methods for the prevention and/or treatment of inflammatory conditions (for example inflammatory bowel diseases (IBD), rheumatoid arthritis, vasculitis, lung diseases (e.g. chronic obstructive pulmonary disease (COPD) and lung interstitial diseases (e.g. idiopathic pulmonary fibrosis (IPF))), neuroinflammatory conditions, infectious diseases, autoimmune diseases, endocrine and/or metabolic diseases, and/or diseases involving impairment of immune cell functions by administering a compound of the invention.根据以下公式Ia的化合物: 其中L1、G和R1如本文所述。 本发明涉及根据公式I的新化合物,这些化合物对抗GPR84,G蛋白偶联受体,该受体参与炎症病症,以及这些新化合物的生产方法,包含这些化合物的药物组合物,以及通过给予本发明的化合物来预防和/或治疗炎症病症(例如炎症性肠病(IBD)、类风湿关节炎、血管炎、肺部疾病(例如慢性阻塞性肺病(COPD)和肺间质疾病(例如特发性肺纤维化(IPF)))、神经炎症病症、传染病、自身免疫疾病、内分泌和/或代谢疾病,以及/或通过给予本发明的化合物导致免疫细胞功能受损的疾病。
-
Efficient synthesis of propargylamines from terminal alkynes, dichloromethane and tertiary amines over silver catalysts作者:Xiuling Chen、Tieqiao Chen、Yongbo Zhou、Chak-Tong Au、Li-Biao Han、Shuang-Feng YinDOI:10.1039/c3ob41878b日期:——A simple, efficient and highly functional group compatible method for the synthesis of propargylamines from terminal alkynes, dichloromethane and tertiary amines using silver catalysts has been developed.
-
[EN] EXPANDED THERAPEUTIC POTENTIAL IN NITROHETEROARYL ANTIMICROBIALS<br/>[FR] POTENTIEL THÉRAPEUTIQUE ÉTENDU DANS DES ANTIMICROBIENS À NITROHÉTÉROARYLE申请人:UNIV CALIFORNIA公开号:WO2014205414A1公开(公告)日:2014-12-24Disclosed herein are antimicrobial compounds compositions, pharmaceutical compositions, the use and preparation thereof. Some embodiments relate to imidazole, thiazole, and furan derivatives and their use as therapeutic agents.
表征谱图
-
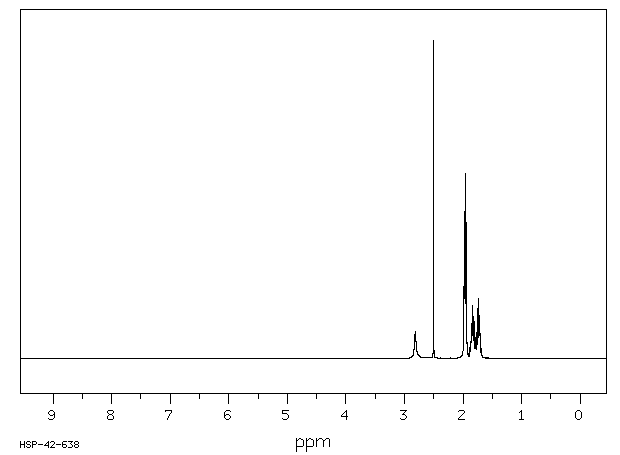
氢谱1HNMR
-
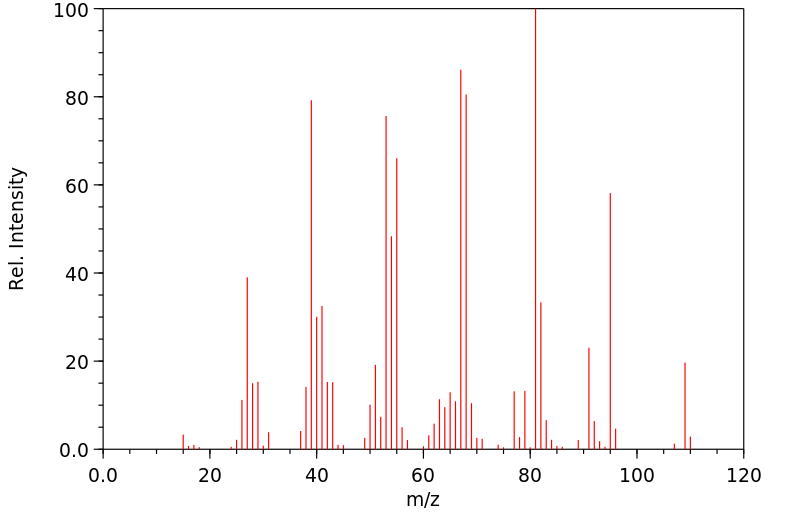
质谱MS
-
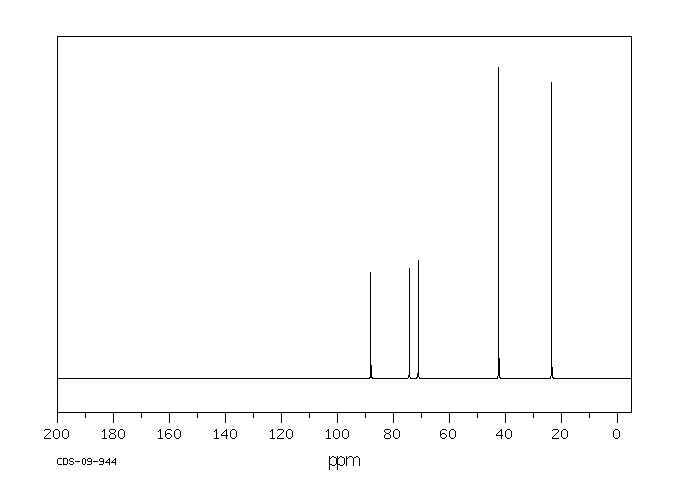
碳谱13CNMR
-
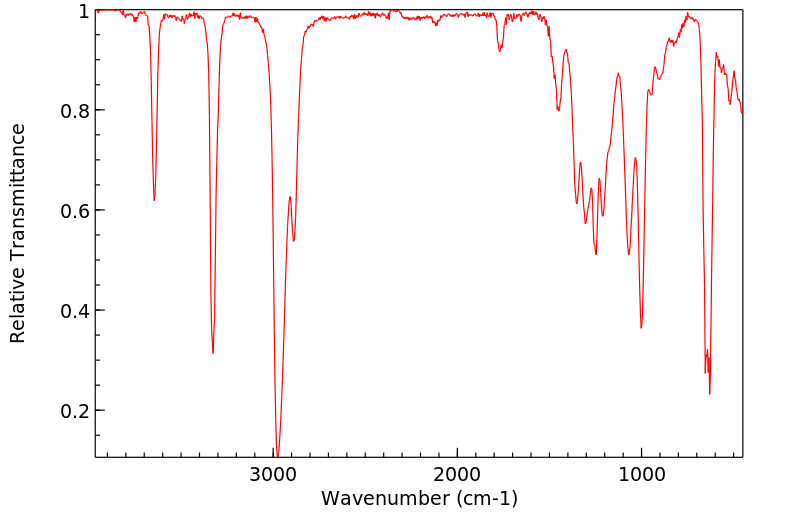
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷