1-氯蒽 | 4985-70-0
中文名称
1-氯蒽
中文别名
——
英文名称
1-chloroanthracene
英文别名
1-Chlor-anthracen
CAS
4985-70-0
化学式
C14H9Cl
mdl
MFCD00003578
分子量
212.678
InChiKey
SRIHSAFSOOUEGL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:77-80 °C (lit.)
-
沸点:275.35°C (rough estimate)
-
密度:1.171 g/mL at 25 °C (lit.)
-
保留指数:1988.7
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险发生。
应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:15
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
WGK Germany:3
-
储存条件:请将药品存放在密闭、阴凉干燥处,并保持良好通风。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 1-Chloroanthracene
CAS-No. : 4985-70-0
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Not a hazardous substance or mixture according to Regulation (EC) No. 1272/2008.
Not a hazardous substance or mixture according to EC-directives 67/548/EEC or 1999/45/EC.
Label elements
The product does not need to be labelled in accordance with EC directives or respective national laws.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C14H9Cl
Molecular Weight : 212,67 g/mol
Section 4. FIRST AID MEASURES
Description of first aid measures
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration.
In case of skin contact
Wash off with soap and plenty of water.
In case of eye contact
Flush eyes with water as a precaution.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water.
Most important symptoms and effects, both acute and delayed
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides, Hydrogen chloride gas
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Avoid dust formation. Avoid breathing vapors, mist or gas.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Sweep up and shovel. Keep in suitable, closed containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
General industrial hygiene practice.
Personal protective equipment
Eye/face protection
Use equipment for eye protection tested and approved under appropriate government standards
such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Choose body protection in relation to its type, to the concentration and amount of dangerous
substances, and to the specific work-place., The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Respiratory protection is not required. Where protection from nuisance levels of dusts are desired,
use type N95 (US) or type P1 (EN 143) dust masks. Use respirators and components tested and
approved under appropriate government standards such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 77 - 80 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,171 g/mL at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. May cause respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. May cause skin irritation.
Eyes May cause eye irritation.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15 - REGULATORY INFORMATION
N/A
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:参考文献:名称:A simple and effective approach for catalytic reductive dechlorination of aromatic compounds摘要:一种高效的制备2(3),9(10),16(17),23(24)-八(正己基)钴(II)酞菁((正己基)8CoPc) (2)的过程被描述。这种新型的钴(II)酞菁通过光谱方法进行表征。它被用作室温下氯化芳香化合物(CACs)的还原脱氯催化剂。结果显示,CACs在110-120分钟内完全脱氯。DOI:10.1139/cjc-2016-0567
-
作为产物:参考文献:名称:轻松合成三蝶烯-唑盐和 NHC-金属配合物摘要:取代的蒽和醌亚胺的环加成是轻松合成新型三蝶烯-NHC-金属配合物的关键步骤。DOI:10.1002/ejic.202300178
文献信息
-
DIRECT ANTI-MARKOVNIKOV ADDITION OF ACIDS TO ALKENES申请人:THE UNIVERSITY OF NORTH CAROLINA AT CHAPEL HILL公开号:US20160096791A1公开(公告)日:2016-04-07A method of making an anti-Markovnikov addition product, comprises reacting an acid with an alkene or alkyne in a dual catalyst reaction system to the exclusion of oxygen to produce said anti-Markovnikov addition product; the dual catalyst reaction system comprising a single electron oxidation catalyst in combination with a hydrogen atom donor catalyst. Dual catalyst composition useful for carrying out such methods are also described.
-
Design of an Indolylphosphine Ligand for Reductive Elimination-Demanding Monoarylation of Acetone Using Aryl Chlorides作者:Wai Chung Fu、Chau Ming So、Wing Kin Chow、On Ying Yuen、Fuk Yee KwongDOI:10.1021/acs.orglett.5b02344日期:2015.9.18elimination-demanding Pd-catalyzed mono-α-arylation of acetone is demonstrated and reported. The catalyst is tolerant of previously proven challenging electron-deficient aryl chlorides and provides excellent product yields with down to 0.1 mol % Pd. Preliminary investigations suggest that the rate-limiting step for the proposed system is the oxidative addition of aryl chlorides, in which it contradicts
-
Efficient Indium-Mediated Dehalogenation of Aromatics in Ionic Liquid Media作者:Álvaro Cañete、Cristian Salas、Flavia ZacconiDOI:10.3390/molecules18010398日期:——An efficient indium-mediated dehalogenation reaction of haloaromatics and haloheteroaromatics in ionic liquids has been studied. This method is simple and effective in the presence of [bmim]Br. Furthermore, this methodology is environmentally friendly compared with conventional ones.
-
Nickel-Catalyzed Cross-Coupling of Organolithium Reagents with (Hetero)Aryl Electrophiles作者:Dorus Heijnen、Jean-Baptiste Gualtierotti、Valentín Hornillos、Ben L. FeringaDOI:10.1002/chem.201505106日期:2016.3.14Nickel‐catalyzed selective cross‐coupling of aromatic electrophiles (bromides, chlorides, fluorides and methyl ethers) with organolithium reagents is presented. The use of a commercially available nickel N‐heterocyclic carbene (NHC) complex allows the reaction with a variety of (hetero)aryllithium compounds, including those prepared via metal‐halogen exchange or direct metallation, whereas a commercially
-
[EN] ANTIMICROBIAL COMPOUNDS, THEIR SYNTHESIS AND APPLICATIONS THEREOF<br/>[FR] COMPOSÉS ANTIMICROBIENS, LEUR SYNTHÈSE ET LEURS APPLICATIONS申请人:JNCASR BANGALORE公开号:WO2014097178A1公开(公告)日:2014-06-26The present disclosure relates to the field of medicinal chemistry and more particularly to the development of antimicrobial compounds. The disclosure relates to the synthesis and characterization of compounds comprising aromatic radical or an aliphatic radical, an alkyl amine and amino acid moiety wherein said compounds exhibit antimicrobial activity against various drug-sensitive and drug-resistant pathogenic 10 microorganisms.
表征谱图
-
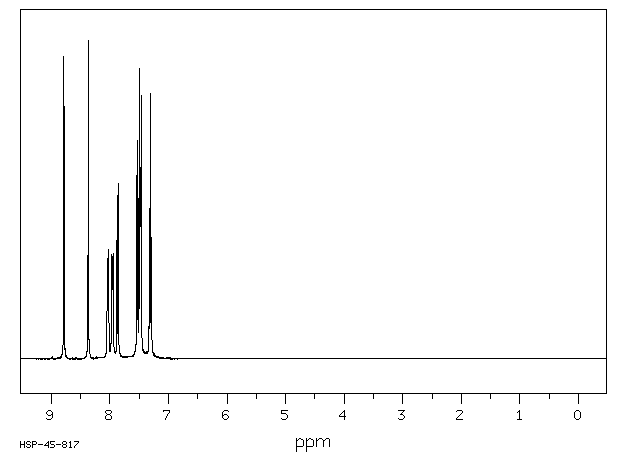
氢谱1HNMR
-
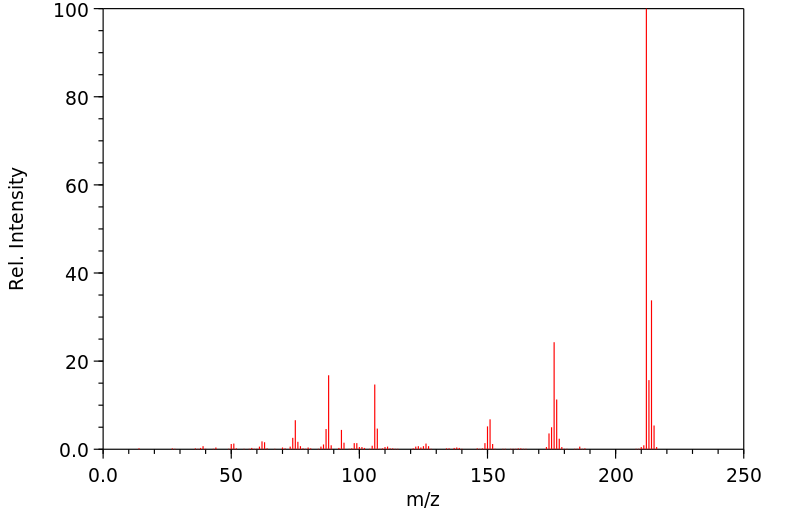
质谱MS
-
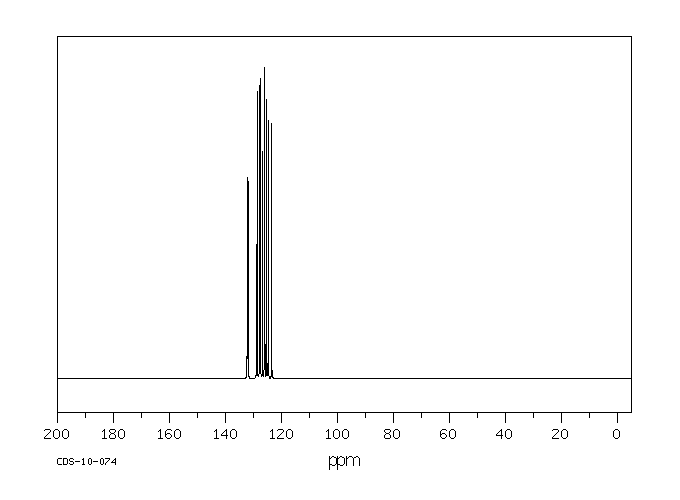
碳谱13CNMR
-
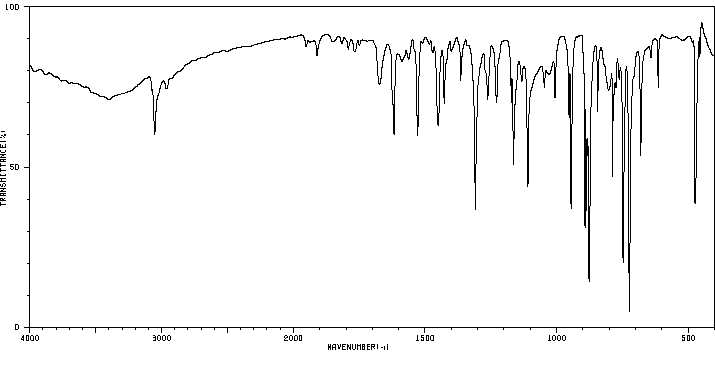
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62