1-碘萘 | 90-14-2
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:4°C
-
沸点:163-165 °C/15 mmHg (lit.)
-
密度:1.74 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
溶解度:0.007克/升
-
介电常数:4.5599999999999996
-
保留指数:1571.9
计算性质
-
辛醇/水分配系数(LogP):4.2
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:29036990
-
危险品运输编号:NONH for all modes of transport
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存储条件:室温、避光且密封保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 1-碘萘
产品名称
1.2 鉴别的其他方法
1-Naphthyl iodide
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
皮肤刺激 (类别 2)
眼睛刺激 (类别 2A)
特异性靶器官系统毒性(一次接触) (类别 3)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防措施
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
事故响应
P302 + P352 如果皮肤接触:用大量肥皂和水清洗。
P304 + P340 如吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如与眼睛接触,用水缓慢温和地冲洗几分钟。如戴隐形眼镜并可方便地取
出,取出隐形眼镜,然后继续冲洗.
P312 如感觉不适,呼救中毒控制中心或医生.
P321 具体处置(见本标签上提供的急救指导)。
P332 + P313 如觉皮肤刺激:求医/就诊。
P337 + P313 如仍觉眼睛刺激:求医/就诊。
P362 脱掉沾污的衣服,清洗后方可再用。
安全储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
废弃处置
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: 1-Naphthyl iodide
别名
: C10H7I
分子式
: 254.07 g/mol
分子量
组分 浓度或浓度范围
1-Iodonaphthalene
<=100%
化学文摘登记号(CAS 90-14-2
No.) 201-968-9
EC-编号
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 向到现场的医生出示此安全技术说明书。
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用大量水彻底冲洗至少15分钟并请教医生。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
咳嗽, 呼吸短促, 头痛, 恶心, 呕吐, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 碘化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
使用个人防护用品。 避免吸入蒸气、烟雾或气体。 保证充分的通风。 人员疏散到安全区域。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
用惰性吸附材料吸收并当作危险废物处理。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 避免吸入蒸气和烟雾。
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据良好的工业卫生和安全规范进行操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
完全接触
物料: 氟橡胶
最小的层厚度 0.7 mm
溶剂渗透时间: 480 min
测试过的物质VitojECt® (KCL 890 / Z677698, 规格 M)
飞溅保护
物料: 氟橡胶
最小的层厚度 0.7 mm
溶剂渗透时间: 480 min
测试过的物质VitojECt® (KCL 890 / Z677698, 规格 M)
, 测试方法 EN374
如果以溶剂形式应用或与其它物质混合应用,或在不同于EN
374规定的条件下应用,请与EC批准的手套的供应商联系。
这个推荐只是建议性的,并且务必让熟悉我们客户计划使用的特定情况的工业卫生学专家评估确认才可.
这不应该解释为在提供对任何特定使用情况方法的批准.
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
如危险性评测显示需要使用空气净化的防毒面具,请使用全面罩式多功能防毒面具(US)或ABEK型
(EN
14387)防毒面具筒作为工程控制的候补。如果防毒面具是保护的唯一方式,则使用全面罩式送风防
毒面具。 呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 透明, 液体
颜色: 深棕
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
163 - 165 °C 在 20 hPa - lit.
g) 闪点
113 °C - 闭杯
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.74 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
咳嗽, 呼吸短促, 头痛, 恶心, 呕吐, 据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
联系专业的拥有废弃物处理执照的机构来处理此物质。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,8-二碘萘 1,8-diiodonaphthalene 1730-04-7 C10H6I2 379.967 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,4-二碘萘 1,4-diiodonaphthalene 36316-83-3 C10H6I2 379.967 2-碘萘 2-iodonaphthalene 612-55-5 C10H7I 254.07 2,6-二-碘萘 2,6-diiodonaphthalene 36316-88-8 C10H6I2 379.967 2,7-二碘萘 2,7-diiodonaphthalene 58556-77-7 C10H6I2 379.967 1-氯-5-碘萘 1-chloro-5-iodonaphthalene 159334-77-7 C10H6ClI 288.515
反应信息
-
作为反应物:描述:参考文献:名称:由芳基卤和醛的钯催化合成的叔丁基胩使用甲酸盐作为氢化物供体†摘要:通过使用叔丁基异氰化物作为C 1资源和甲酸盐作为氢化物供体,无需任何其他碱,就已经实现了芳烃卤化物的高效一锅钯催化的芳基卤化物的加氢甲酰化反应。反应条件温和,易于操作且毒性较低,该反应可耐受各种官能团,产率中等至优异。DOI:10.1039/c4ra16388e
-
作为产物:参考文献:名称:无配体铜(0)催化的C−S乌尔曼型交叉偶联反应:5,4-二取代的2,4-二氢-3H-1,2,4-三唑-3-硫酮的S-芳基化摘要:在无配体 Cu(0) 催化的 Ullmann 型反应条件下研究了 5,4-二取代 2,4-二氢-3H-1,2,4-三唑-3-硫酮的芳基化反应,结果表明该反应以化学选择性进行并形成S-芳基化产物。在一些情况下,当芳基化剂中存在吸电子基团时,反应可以在没有催化剂的情况下进行。DOI:10.1002/ejoc.202400199
-
作为试剂:描述:乙烯基三甲基硅烷 在 bis(η3-allyl-μ-chloropalladium(II)) 1-碘萘 、 水 作用下, 以 1,2,3,4-四氢萘 、 N,N-二甲基甲酰胺 为溶剂, 反应 5.0h, 生成 1-乙烯萘 、 碘代三甲硅烷 、 六甲基二硅氧烷 、 1,3-丁二烯参考文献:名称:Reaction of combination of trimethylvinylsilane with organic halides catalyzed by palladium derivatives摘要:DOI:10.1007/bf00954099
文献信息
-
A mild and efficient copper-catalyzed coupling of aryl iodides and thiols using an oxime–phosphine oxide ligand作者:Di Zhu、Lei Xu、Fan Wu、Boshun WanDOI:10.1016/j.tetlet.2006.05.178日期:2006.8A mild and efficient copper-catalyzed system for the coupling of aryl iodides and thiols was developed using a readily prepared and highly stable oxime–phosphine oxide ligand. Good to excellent yields were obtained.
-
Microwave-Assisted Simple and Efficient Ligand Free Copper Nanoparticle Catalyzed Aryl-Sulfur Bond Formation作者:Brindaban C. Ranu、Amit Saha、Ranjan JanaDOI:10.1002/adsc.200700289日期:2007.12.10A new protocol for the coupling of aryl iodides with thiophenols and alkanethiols catalyzed by copper nanoparticles under ligand-free condition has been developed. A variety of functionalized aryl sulfides are prepared in excellent yields under microwave irradiation for 5–7 min. A plausible radical mechanism has been suggested.
-
Regioselective Arylation Reactions of Biphenyl-2-ols, Naphthols, and Benzylic Compounds with Aryl Halides under Palladium Catalysis作者:Tetsuya Satoh、Jun-ichi Inoh、Yoshiki Kawamura、Yuichiro Kawamura、Masahiro Miura、Masakatsu NomuraDOI:10.1246/bcsj.71.2239日期:1998.9Biphenyl-2-ols undergo regioselective mono- and diarylation upon a treatment with aryl iodides in the presence of a palladium catalyst in DMF using Cs2CO3 as a base to produce 1,1′ : 2′,1″-terphenyl-2-ol and 2′,6′-diphenylbiphenyl-2-ol and their derivatives. The reaction of 1-naphthol selectively occurs at its 8-position to give 8-aryl-1-naphthols. In the reaction of 2-naphthol with aryl bromides, diarylated
-
Valorisation of urban waste to access low-cost heterogeneous palladium catalysts for cross-coupling reactions in biomass-derived γ-valerolactone作者:Federica Valentini、Francesco Ferlin、Simone Lilli、Assunta Marrocchi、Liu Ping、Yanlong Gu、Luigi VaccaroDOI:10.1039/d1gc01707a日期:——commercial Pd/C catalyst. The good reactivity in the biomass-derived solvent (GVL) confirms that the Pd/PiNe heterogeneous catalyst is a valid system that can be integrated into a waste valorization chain within a circular economy approach. In addition, the efficiency of the catalyst has also been extended to perform the challenging consecutive Hiyama–Heck reaction to afford differently substituted (E)-1在这里,我们报告了一个简单的协议,用于评估普通城市生物废物的价值。将松针城市垃圾预处理后获得的木质纤维素生物质有效地转化为用于固定 Pd 纳米颗粒的低成本载体 (PiNe)。最终的 Pd/PiNe 多相催化剂具有小粒径 (4.5 nm) 和金属负载 (9.9 wt%),可与大多数市售和常用的对应物相媲美。在这项贡献中,我们测试了 Pd/PiNe 系统在两个代表性交叉偶联 Heck 和 Hiyama 反应中的催化效率,并比较了与商业 Pd/C 催化剂获得的结果。生物质衍生溶剂 (GVL) 中的良好反应性证实了 Pd/PiNe 多相催化剂是一种有效的系统,可以在循环经济方法中整合到废物价值链中。此外,催化剂的效率也得到了扩展,以进行具有挑战性的连续 Hiyama-Heck 反应,以提供不同取代的(E )-1,2-二芳基乙烯。
-
Efficient one-pot transformation of aminoarenes to haloarenes using halodimethylisulfonium halides generated in situ作者:Woonphil Baik、Wanqiang Luan、Hyun Joo Lee、Cheol Hun Yoon、Sangho Koo、Byeong Hyo KimDOI:10.1139/v05-026日期:2005.3.1
Halodimethylsulfonium halide 1, which is readily formed in situ from hydrohaloic acid and DMSO, is a good nucleophilic halide. This activated nucleophilic halide rapidly converts aryldiazonium salt prepared in situ by the same hydrohaloic acid and nitrite ion to aryl chlorides, bromides, or iodides in good yield. The combined action of nitrite ion and hydrohaloic acid in DMSO is required for the direct transformation of aromatic amines, which results in the production of aryl halides within 1 h. Substituted compounds with electron-donating or -withdrawing groups or sterically hindered aromatic amines are also smoothly transformed to the corresponding aromatic halides. The only observed by-product is the deaminated arene (usually <7%). The isolated aryldiazonium salts can also be converted to the corresponding aryl halides using 1. The present method offers a facile, one-step procedure for transforming aminoarenes to haloarenes and lacks the environmental pollutants that usually accompany the Sandmeyer reaction using copper halides. Key words: aminoarenes, haloarenes, halodimethylsulfonium halide, halogenation, amination.
卤二甲基亚砜卤化物1是一种良好的亲核卤化物,可在现场由氢卤酸和二甲亚砜形成。这种活化的亲核卤化物迅速将由相同的氢卤酸和亚硝酸根在现场制备的芳基重氮盐转化为芳基氯化物、溴化物或碘化物,收率较高。在DMSO中,亚硝酸根和氢卤酸的联合作用是直接转化芳香胺的必要条件,从而在1小时内产生芳基卤化物。带有电子给体或吸引基团或有立体位阻的芳香胺的取代化合物也可顺利转化为相应的芳香卤化物。观察到的唯一副产物是去氨基芳烃(通常<7%)。孤立的芳基重氮盐也可以使用1转化为相应的芳基卤化物。该方法提供了一种简便的、一步法的程序,用于将氨基芳烃转化为卤代芳烃,并且不伴随通常伴随使用铜卤化物进行桑迈尔反应的环境污染物。关键词:氨基芳烃,卤代芳烃,卤二甲基亚砜卤化物,卤化,胺化。
表征谱图
-
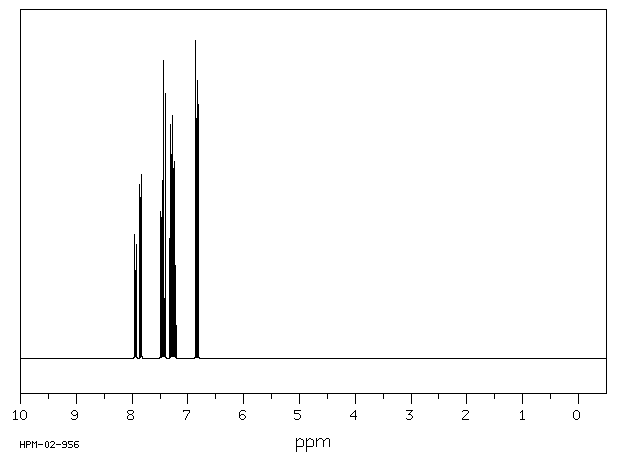
氢谱1HNMR
-
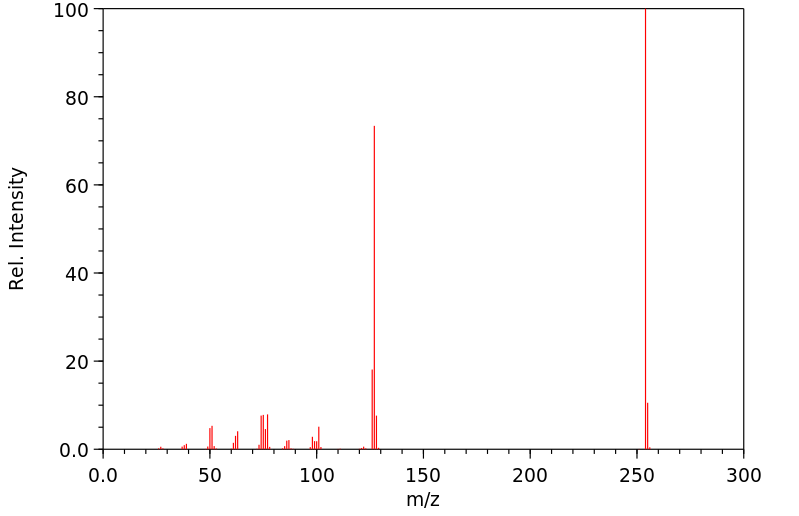
质谱MS
-
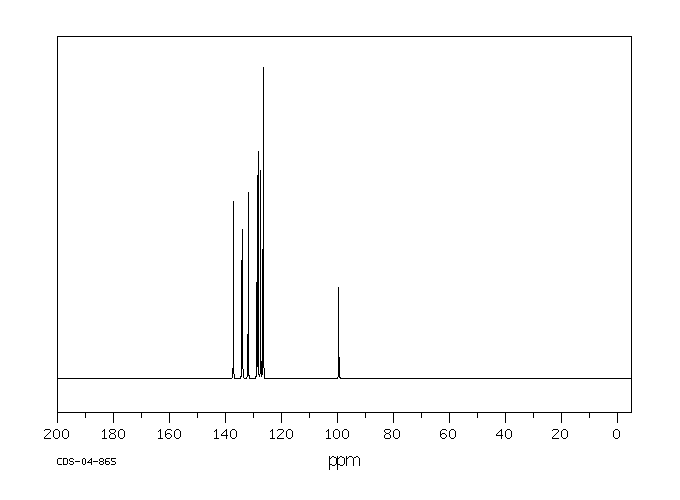
碳谱13CNMR
-
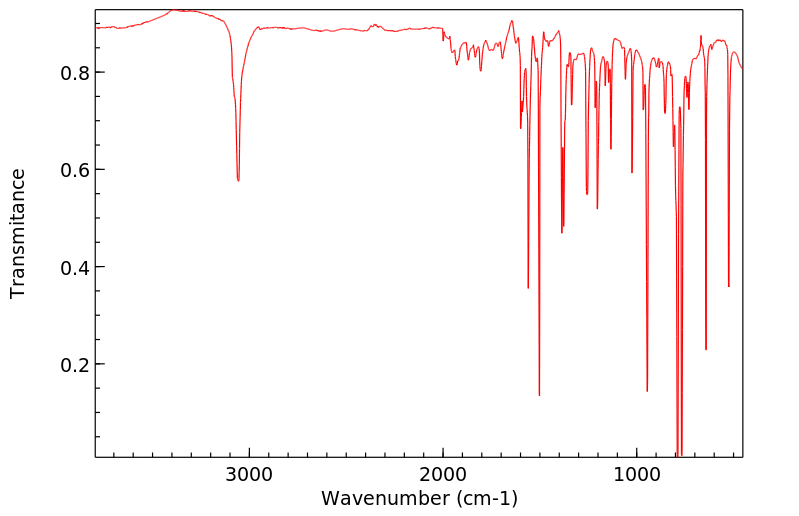
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息