3,4-(亚甲二氧基)甲苯 | 7145-99-5
分子结构分类
中文名称
3,4-(亚甲二氧基)甲苯
中文别名
5-甲基-1,3-苯并二噁茂;3,4-(亚甲二氧)甲苯;5-甲基-1,3-苯并间二氧杂环戊二烯
英文名称
5-methyl-1,3-benzodioxole
英文别名
3,4-(methylenedioxy)toluene;5-methylbenzo[d][1,3]dioxole;1-methyl-3,4-methylenedioxybenzene
CAS
7145-99-5
化学式
C8H8O2
mdl
MFCD00005833
分子量
136.15
InChiKey
GHPODDMCSOYWNE-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:199-200 °C (lit.)
-
密度:1.135 g/mL at 25 °C (lit.)
-
闪点:169 °F
-
稳定性/保质期:
遵照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
危险类别码:R20/21/22,R36/37/38
-
WGK Germany:3
-
RTECS号:DF4921090
-
安全说明:S23,S24/25
-
包装等级:III
-
危险类别:8
-
危险性防范说明:P280,P305+P351+P338
-
危险品运输编号:1760
-
危险性描述:H302,H318
-
储存条件:密封于阴凉干燥处。
SDS
SECTION 1: Identification of the substance/mixture and of the company/undertaking
Product identifiers
Product name : 3,4-(Methylenedioxy)toluene
REACH No. : A registration number is not available for this substance as the substance
or its uses are exempted from registration, the annual tonnage does not
require a registration or the registration is envisaged for a later
registration deadline.
CAS-No. : 7145-99-5
SECTION 2: Hazards identification
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008
Acute toxicity, Oral (Category 4), H302
Serious eye damage (Category 1), H318
For the full text of the H-Statements mentioned in this Section, see Section 16.
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Xn Harmful R22, R41
For the full text of the R-phrases mentioned in this Section, see Section 16.
Label elements
Labelling according Regulation (EC) No 1272/2008
Pictogram
Signal word Danger
Hazard statement(s)
Harmful if swallowed.
Causes serious eye damage.
Precautionary statement(s)
Wear protective gloves/ eye protection/ face protection.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
Other hazards - none
SECTION 3: Composition/information on ingredients
Substances
Synonyms : 5-Methyl-1,3-benzodioxole
Formula : C8H8O2
Molecular Weight : 136,15 g/mol
CAS-No. : 7145-99-5
EC-No. : 230-453-1
Hazardous ingredients according to Regulation (EC) No 1272/2008
Component Classification Concentration
5-Methyl-1,3-benzodioxole
CAS-No. 7145-99-5 Acute Tox. 4; Eye Dam. 1; <= 100 %
EC-No. 230-453-1 H302, H318
Hazardous ingredients according to Directive 1999/45/EC
Component Classification Concentration
5-Methyl-1,3-benzodioxole
CAS-No. 7145-99-5 Xn, R22 - R41 <= 100 %
EC-No. 230-453-1
For the full text of the H-Statements and R-Phrases mentioned in this Section, see Section 16
SECTION 4: First aid measures
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Do NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with
water. Consult a physician.
Most important symptoms and effects, both acute and delayed
The most important known symptoms and effects are described in the labelling (see section 2.2) and/or in
section 11
Indication of any immediate medical attention and special treatment needed
no data available
SECTION 5: Firefighting measures
Extinguishing media
Suitable extinguishing media
For small (incipient) fires, use media such as "alcohol" foam, dry chemical, or carbon dioxide. For large
fires, apply water from as far as possible. Use very large quantities (flooding) of water applied as a mist or
spray; solid streams of water may be ineffective. Cool all affected containers with flooding quantities of
water.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
Use water spray to cool unopened containers.
SECTION 6: Accidental release measures
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid breathing vapours, mist or gas. Ensure adequate ventilation.
Remove all sources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to
form explosive concentrations. Vapours can accumulate in low areas.
For personal protection see section 8.
Environmental precautions
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into the
environment must be avoided.
Methods and materials for containment and cleaning up
Contain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and
place in container for disposal according to local regulations (see section 13). Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
SECTION 7: Handling and storage
Precautions for safe handling
Avoid contact with skin and eyes. Avoid inhalation of vapour or mist.
Keep away from sources of ignition - No smoking.Take measures to prevent the build up of electrostatic
charge.
For precautions see section 2.2.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place. Containers which are
opened must be carefully resealed and kept upright to prevent leakage.
Specific end use(s)
A part from the uses mentioned in section 1.2 no other specific uses are stipulated
SECTION 8: Exposure controls/personal protection
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Tightly fitting safety goggles. Faceshield (8-inch minimum). Use equipment for eye protection
tested and approved under appropriate government standards such as NIOSH (US) or EN
166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
Complete suit protecting against chemicals, The type of protective equipment must be selected
according to the concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
Where risk assessment shows air-purifying respirators are appropriate use a full-face respirator
with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup
to engineering controls. If the respirator is the sole means of protection, use a full-face supplied air
respirator. Use respirators and components tested and approved under appropriate government
standards such as NIOSH (US) or CEN (EU).
Control of environmental exposure
Prevent further leakage or spillage if safe to do so. Do not let product enter drains. Discharge into
the environment must be avoided.
SECTION 9: Physical and chemical properties
Information on basic physical and chemical properties
a) Appearance Form: liquid
Colour: light yellow
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing no data available
point
f) Initial boiling point and 199 - 200 °C - lit.
boiling range
g) Flash point 76 °C - closed cup
h) Evapouration rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density 1,135 g/cm3 at 25 °C
n) Water solubility no data available
o) Partition coefficient: n- log Pow: 2,46
octanol/water
p) Auto-ignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
SECTION 10: Stability and reactivity
Reactivity
no data available
Chemical stability
Stable under recommended storage conditions.
Possibility of hazardous reactions
no data available
Conditions to avoid
Heat, flames and sparks.
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
In the event of fire: see section 5
SECTION 11: Toxicological information
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitisation
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
no data available
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Additional Information
RTECS: DF4921090
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
SECTION 12: Ecological information
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
PBT/vPvB assessment not available as chemical safety assessment not required/not conducted
Other adverse effects
Harmful to aquatic life.
SECTION 13: Disposal considerations
Waste treatment methods
Product
This combustible material may be burned in a chemical incinerator equipped with an afterburner and
scrubber. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material.
Contaminated packaging
Dispose of as unused product.
SECTION 14: Transport information
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
SECTION 15: Regulatory information
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
For this product a chemical safety assessment was not carried out
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 胡椒醇 piperonol 495-76-1 C8H8O3 152.15 5-溴甲基-苯并[1,3]二氧代 3,4-Methylenedioxybenzyl bromide 2606-51-1 C8H7BrO2 215.046 1,3-苯并二氧杂环戊烯-5-乙腈 1,3-benzodioxole-5-acetonitrile 4439-02-5 C9H7NO2 161.16 —— (E)-1,3-benzodioxol-5-ylmethylidenehydrazine —— C8H8N2O2 164.16 —— (benzo[d][1,3]dioxol-5-ylmethylene)hydrazine 61428-01-1 C8H8N2O2 164.164 胡椒乙酸 1,3-benzodioxole-5-acetic acid 2861-28-1 C9H8O4 180.16 胡椒酸 Piperonylic acid 94-53-1 C8H6O4 166.133 1,3-苯并二氧戊环-5-甲醛缩氨基脲 3,4-methylenedioxybenzaldehyde semicarbazone 16742-62-4 C9H9N3O3 207.189 —— 2-[(Benzo[1,3]dioxol-5-ylmethyl)-amino]-2-methyl-propan-1-ol 87306-69-2 C12H17NO3 223.272 洋茉莉醛二乙缩醛 piperonal diethylacetal 40527-42-2 C12H16O4 224.257 N-胡椒基苯胺 N-(benzo[d][1,3]dioxol-5-ylmethyl)aniline 3526-42-9 C14H13NO2 227.263 甲基 1,3-苯并二恶唑-5-羧酸酯 methyl 3,4-methylenedioxybenzoate 326-56-7 C9H8O4 180.16 —— 5-[1,3]dithiolan-2-yl-benzo[1,3]dioxole 5769-01-7 C10H10O2S2 226.32 芝麻酚 Sesamol 533-31-3 C7H6O3 138.123 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 胡椒醛 piperonal 120-57-0 C8H6O3 150.134 5-溴甲基-苯并[1,3]二氧代 3,4-Methylenedioxybenzyl bromide 2606-51-1 C8H7BrO2 215.046 胡椒基氯 5-(chloromethyl)-1,3-benzodioxole 20850-43-5 C8H7ClO2 170.595 3,4-二甲氧基甲苯 1,2-dimethoxy-4-methylbenzene 494-99-5 C9H12O2 152.193 —— (±)-2-methoxy-5-methyl-1,3-benzodioxole 96963-58-5 C9H10O3 166.177 —— piperonyl dibromide —— C8H6Br2O2 293.942 —— 3-(3,4-methylenedioxyphenyl)-1-propyne 133218-07-2 C10H8O2 160.172 —— 2-methyl-4,5-methylenedioxybenzyl alcohol 675842-22-5 C9H10O3 166.177 6-甲基-1,3-苯并二氧-5-甲醛 6-methylbenzo[d][1,3]dioxole-5-carbaldehyde 58343-54-7 C9H8O3 164.161 —— 2-methyl-4,5-methylenedioxyphenylethene 891193-05-8 C10H10O2 162.188 (3,4-亚甲基二氧基)-6-甲基苄氯 5-(chloromethyl)-6-methylbenzo[1,3]dioxole 117661-72-0 C9H9ClO2 184.622 —— d3-5-methylbenzo[d][1,3]dioxole 1071170-18-7 C8H8O2 139.126 2,2-二氯-5-甲基苯并[d][1,3]二氧杂环戊烯 2,2-dichloro-5-methyl-1,3-benzodioxole 68119-28-8 C8H6Cl2O2 205.04 2,2-二氟-5-甲基苯并d1,3二氧代 2,2-difluoro-5-methyl-1,3-benzodioxole 68119-29-9 C8H6F2O2 172.131 2-乙氧基-4-甲基苯酚 2-ethoxy-4-methylphenol 2563-07-7 C9H12O2 152.193 胡椒酸 Piperonylic acid 94-53-1 C8H6O4 166.133 —— 2-ethoxy-5-methylphenol 2563-04-4 C9H12O2 152.193 5-溴-6-甲基-1,3-苯并二氧戊环 5-bromo-6-methylbenzo[d][1,3]dioxole 5025-53-6 C8H7BrO2 215.046 —— 2-(6-methylbenzo[d][1,3]dioxol-5-yl)ethan-1-amine 117661-79-7 C10H13NO2 179.219 —— 4,5-methylenedioxy-2-methylbenzyl cyanide 67301-90-0 C10H9NO2 175.187 —— 2-iodo-4,5-methylenedioxytoluene 60229-63-2 C8H7IO2 262.047 6-甲基-1,3-苯并二氧代l-5-胺 2-Amino-4,5-methylendioxy-toluol 62052-49-7 C8H9NO2 151.165 —— 5-((phenylthio)methyl)benzo[d][1,3]dioxole 103844-15-1 C14H12O2S 244.314 5-(溴甲基)-2,2-二氟-2H-1,3-苯并二恶唑 5-(bromomethyl)-2,2-difluorobenzo[d][1,3]dioxole 68119-30-2 C8H5BrF2O2 251.027 —— bis-(6-methyl-benzo[1,3]dioxol-5-yl)-methane 109733-91-7 C17H16O4 284.312 (2,2-二氟-苯并[1,3]二氧代l-5-基)-乙腈 2-(2,2-difluorobenzo[d][1,3]dioxol-5-yl)acetonitrile 68119-31-3 C9H5F2NO2 197.141 —— (3E)-4-(2-methyl-4,5-methylenedioxyphenyl)-3-buten-2-one —— C12H12O3 204.225 6-甲基苯并[D][1,3]1,3-二氧杂环戊烯-5-羧酸 2-Methyl-4,5-methylendioxy-benzoesaeure 5025-54-7 C9H8O4 180.16 —— (E)-1-(2-methyl-4,5-methylenedioxyphenyl)-2-(2-hydroxyphenyl)ethene 891193-07-0 C16H14O3 254.285 1-(6-甲基-1,3-苯并二氧戊环-5-基)乙酮 1-(6-methyl-2H-1,3-benzodioxol-5-yl)ethan-1-one 96543-89-4 C10H10O3 178.18 —— methyl (E)-4-(6-methylbenzo[d][1,3]dioxol-5-yl)but-2-enoate 1415817-61-6 C13H14O4 234.252 3,4-二羟基甲苯 4-Methylcatechol 452-86-8 C7H8O2 124.139 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:描述:3,4-(亚甲二氧基)甲苯 在 盐酸 、 聚合甲醛 、 四丁基溴化铵 、 magnesium 作用下, 以 四氢呋喃 、 水 为溶剂, 反应 1.55h, 生成 N-(4-氯-3-甲基-5-异恶唑基)-2-[(2-甲基-4,5-亚甲二氧基苯基)乙酰]噻吩-3-磺酰胺参考文献:名称:WO2007/106494摘要:公开号:
-
作为产物:参考文献:名称:用于羰基和醇化学选择性加氢脱氧的金属-有机骨架限制的单中心基金属催化剂摘要:通过多相贱金属催化剂使用氢对羰基和醇进行化学选择性脱氧对于精细化学品和生物燃料的可持续生产至关重要。我们报告了一种铝金属有机骨架 (DUT-5) 节点负载钴 (II) 氢化物,它是一种高度化学选择性和可回收的多相催化剂,用于一系列芳香族和脂肪族酮、醛以及伯和仲醇的脱氧,包括1 bar H 2下的生物质衍生底物。通过用 CoCl 2对 DUT-5 的二级构建单元 (SBU) 进行后合成金属化,然后与 NaEt 3反应,可以轻松制备单点钴催化剂 (DUT-5-CoH)BH。X 射线光电子能谱和 X 射线吸收近边光谱 (XANES) 表明催化后 DUT-5-CoH 和 DUT-5-Co 中存在 Co II和 Al III中心。通过扩展X射线精细结构光谱(EXAFS)和密度泛函理论建立了催化前后DUT-5-Co钴中心的配位环境。动力学和计算数据表明,在转换限制步骤之前,羰基与钴的可逆配位,包括配位的羰基DOI:10.1021/acs.inorgchem.1c01008
-
作为试剂:描述:1-O-allyl-4,5-di-O-benzyl-3-O-(4-(3,4-dimethoxyphenyl)benzyl)-6-O-(2,3,4,6-tetra-O-benzyl-α-D-mannopyranosyl)-2-O-(2,3,4-tri-O-benzyl-6-O-hexadecanoyl-α-D-mannopyranosyl)-D-myo-inositol 在 3,4-(亚甲二氧基)甲苯 、 三氟乙酸 作用下, 以 二氯甲烷 为溶剂, 反应 1.0h, 以74%的产率得到1-O-allyl-4,5-di-O-benzyl-6-O-(2,3,4,6-tetra-O-benzyl-α-D-mannopyranosyl)-2-O-(2,3,4-tri-O-benzyl-6-O-palmitoyl-α-D-mannopyranosyl)-D-myo-inositol参考文献:名称:分枝杆菌磷脂酰肌醇及其二甘露糖苷的合成及质谱表征摘要:准备一个均具有主要的天然19:0/16:0磷脂酰酰化模式的天然分枝杆菌磷脂酰肌醇(PI)及其二甘露糖苷(PIM 2,AcPIM 2和Ac 2 PIM 2)家族,以研究其质谱碎裂。其中,完全脂化的PIM(即(16:0,18:0)(19:0/16:0)-PIM 2)的首次合成是由(±)-1,2:4,5实现的-diisopropylidene- d -肌醇肌醇在3%总产率16个步骤。该策略的关键特征是扩展了对-(3,4-二甲氧基苯基)苄基保护基在O上的应用。-3位肌醇允许在合成的后期安装硬脂酰残基。对合成的PIM进行了质谱研究,并将其与从M.的脂质提取物中鉴定出的天然PIM的报道进行了比较。牛眼BCG。这些分析证实,片段化模式可用于从细胞壁脂质提取物中鉴定特定PIM的结构。DOI:10.1021/jo301189y
文献信息
-
[EN] PYRAZOLE DERIVATIVES USEFUL AS INHIBITORS OF FAAH<br/>[FR] DÉRIVÉS DE PYRAZOLE UTILES COMME INHIBITEURS DE FAAH申请人:MERCK & CO INC公开号:WO2009151991A1公开(公告)日:2009-12-17The present invention is directed to certain imidazole derivatives which are useful as inhibitors of Fatty Acid Amide Hydrolase (FAAH). The invention is also concerned with pharmaceutical formulations comprising these compounds as active ingredients and the use of the compounds and their formulations in the treatment of certain disorders, including osteoarthritis, rheumatoid arthritis, diabetic neuropathy, postherpetic neuralgia, skeletomuscular pain, and fibromyalgia, as well as acute pain, migraine, sleep disorder, Alzheimer disease, and Parkinson's disease
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] HETEROCYCLIC COMPOUNDS FOR THE TREATMENT OF STRESS-RELATED CONDITIONS<br/>[FR] COMPOSÉS HÉTÉROCYCLIQUES POUR LE TRAITEMENT D'ÉTATS LIÉS AU STRESS申请人:OTSUKA PHARMA CO LTD公开号:WO2010137738A1公开(公告)日:2010-12-02The present invention provides a novel heterocyclic compound. A heterocyclic compound represented by general formula (1) wherein, R1 and R2, each independently represent hydrogen; a phenyl lower alkyl group that may have a substituent(s) selected from the group consisting of a lower alkyl group and the like on a benzene ring and/or a lower alkyl group; or a cyclo C3-C8 alkyl lower alkyl group; or the like; R3 represents a lower alkynyl group or the like; R4 represents a phenyl group that may have a substituent(s) selected from the group consisting of a 1,3,4-oxadiazolyl group that may have e.g., halogen or a heterocyclic group selected from pyridyl group and the like; the heterocyclic group may have at least one substituent(s) selected from a lower alkoxy group and the like or a salt thereof.
-
INDANYLOXYDIHYDROBENZOFURANYLACETIC ACIDS申请人:ECKHARDT Matthias公开号:US20140163025A1公开(公告)日:2014-06-12The present invention relates to compounds of general formula I, wherein the groups (Het)Ar and R 1 are defined as in claim 1 , which have valuable pharmacological properties, in particular bind to the GPR40 receptor and modulate its activity. The compounds are suitable for treatment and prevention of diseases which can be influenced by this receptor, such as metabolic diseases, in particular diabetes type 2.本发明涉及一般式I的化合物, 其中基团(Het)Ar和R1如权利要求1所定义, 具有有价值的药理特性,特别是结合GPR40受体并调节其活性。这些化合物适用于治疗和预防可以受到该受体影响的疾病,如代谢性疾病,特别是2型糖尿病。
-
HETEROCYCLIC INHIBITORS OF ERK1 AND ERK2 AND THEIR USE IN THE TREATMENT OF CANCER申请人:Asana Biosciences, LLC公开号:US20160362407A1公开(公告)日:2016-12-15The present application provides novel heterocyclic compounds and pharmaceutically acceptable salts thereof. Also provided are methods for preparing these compounds. These compounds are useful for inhibiting ERK1/2. By administering to a patient in need a therapeutically effective amount of one or more of the compounds of formula (I), wherein X, Y, Z, J, M, and R 1 to R 8 are defined herein, these compounds are effective in treating conditions associated with dysregulation of the RAS/RAF/MEK/ERK pathway. A variety of conditions can be treated using these compounds and include diseases which are characterized by abnormal cellular proliferation. In one embodiment, the disease is cancer.本申请提供了新颖的杂环化合物及其药用可接受的盐。还提供了制备这些化合物的方法。这些化合物对抑制ERK1/2有用。通过向需要治疗的患者施用式(I)的一个或多个化合物的治疗有效量,其中X、Y、Z、J、M和R1至R8在此处定义,这些化合物在治疗与RAS/RAF/MEK/ERK通路失调相关的疾病方面是有效的。可以使用这些化合物治疗各种疾病,包括以异常细胞增殖为特征的疾病。在一个实施例中,该疾病是癌症。
表征谱图
-
氢谱1HNMR
-
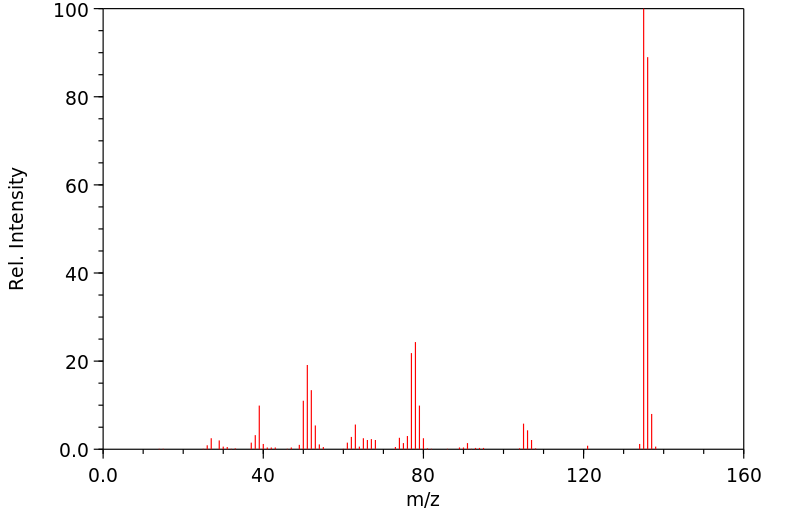
质谱MS
-
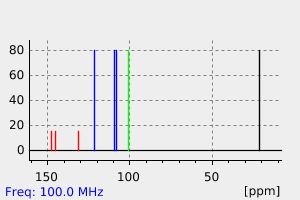
碳谱13CNMR
-
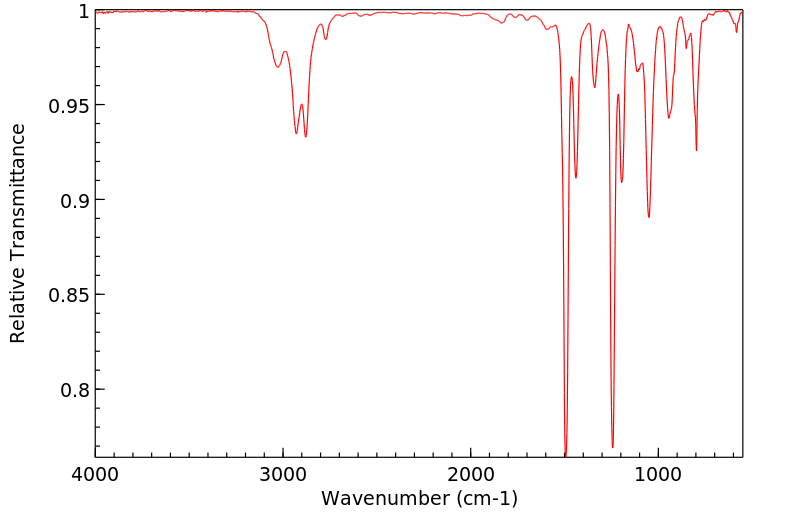
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5-(4-乙氧基-3-甲基苄基)-1,3-苯并二恶茂)
黄樟素氧化物
黄樟素乙二醇; 2',3'-二氢-2',3'-二羟基黄樟素
黄樟素
风藤酰胺
风藤酮
非哌西特盐酸盐
非哌西特 盐酸盐
角秋水仙碱
螺[1,3-苯并二氧戊环-2,1'-环己烷]-5-胺
蓝细菌
苯并[d][1,3]二氧杂环戊烯-5-胺盐酸盐
苯并[d][1,3]二氧代l-5-甲基(2-氧代乙基)氨基甲酸叔丁酯
苯并[d][1,3]二氧代l-5-氨基甲酸叔丁酯
苯并[d][1,3]二氧代-4-甲腈
苯并[d][1,3]二氧代-4-氨基甲酸叔丁酯
苯并[d[1,3]二氧代-4-羧酰胺
苯并[1,3]二氧杂环戊烯-5-基甲基2-氯乙酸酯
苯并[1,3]二氧杂环戊烯-5-基甲基-苄基-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-[2-(4-氟-苯基)-乙基]-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(四氢-呋喃-2-基甲基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(2-氟-苄基)-胺
苯并[1,3]二氧杂环戊烯-5-基甲基-(1-甲基-哌啶-4-基)-胺
苯并[1,3]二氧代l-5-甲基-吡啶-3-甲基-胺
苯并[1,3]二氧代l-5-甲基-(4-氟-苄基)-胺
苯并[1,3]二氧代l-5-乙酸甲酯
苯并[1,3]二氧代-5-羧酰胺盐酸盐
苯并[1,3]二氧代-5-甲基肼盐酸盐
苯并[1,3]二氧代-5-甲基吡啶-4-甲胺
苯并[1,3]二氧代-5-甲基-吡啶-2-甲胺
苯并[1,3]二氧代-5-乙酰氯
苯并-1,3-二氧杂环戊烯-5-甲醇丙酸酯
苯乙酸,1-(1,3-苯并二氧杂环戊烯-5-基)-3-丁烯-1-基酯
苯乙酮O-((4-(3,4-亚甲二氧基苄基)-1-哌嗪-1-基)羰基甲基)肟
苯,1-甲氧基-6-硝基-3,4-亚甲二氧基-
芝麻酚
芝麻林素
脲,N-1,3-苯并二噁唑-5-基-N'-(2-溴乙基)-
胡椒醛肟
胡椒醛-((Z)-O-苯基氨基甲酰基肟)
胡椒醛,二苄基缩硫醛
胡椒醛
胡椒醇
胡椒酸酰氯
胡椒酸
胡椒腈
胡椒环乙酮肟
胡椒环
胡椒基重氮酮
胡椒基甲醛