3-(苯磺酰)丙酸 | 10154-71-9
物质功能分类
中文名称
3-(苯磺酰)丙酸
中文别名
——
英文名称
3-(phenylsulfonyl)propionic acid
英文别名
3-(phenylsulfonyl)propanoic acid;3-benzenesulfonyl propionic acid;3-Benzolsulfonyl-propionsaeure;β-Phenylsulfonyl-propionsaeure;3-Phenylsulfonyl-propionsaeure;3-(benzenesulfonyl)propanoic acid
CAS
10154-71-9
化学式
C9H10O4S
mdl
MFCD00010141
分子量
214.242
InChiKey
WGTYYNCSWCKXAI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:128-130 °C (lit.)
-
沸点:458.9±37.0 °C(Predicted)
-
密度:1.348±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇,溶解度25mg/mL,透明,无色
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险发生。
应避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.222
-
拓扑面积:79.8
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
WGK Germany:3
-
海关编码:2916399090
-
危险类别:IRRITANT
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在密闭、阴凉干燥处,并保持良好通风。
SDS
1.1 产品标识符
: 3-(Phenylsulfonyl)propionic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H10O4S
分子式
: 214.24 g/mol
分子量
成分 浓度
3-(Phenylsulfonyl)propionic acid
-
化学文摘编号(CAS No.) 10154-71-9
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 128 - 130 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 3-(Phenylsulfonyl)propionic acid
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
当心 - 物质尚未完全测试。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C9H10O4S
分子式
: 214.24 g/mol
分子量
成分 浓度
3-(Phenylsulfonyl)propionic acid
-
化学文摘编号(CAS No.) 10154-71-9
模块 4. 急救措施
4.1 必要的急救措施描述
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。
在皮肤接触的情况下
用肥皂和大量的水冲洗。
在眼睛接触的情况下
用水冲洗眼睛作为预防措施。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。
4.2 最重要的症状和影响,急性的和滞后的
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 硫氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
防止粉尘的生成。 防止吸入蒸汽、气雾或气体。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
人身保护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 128 - 130 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3-(phenylsulfonyl)propanoate 10154-72-0 C10H12O4S 228.269 —— 3-benzenesulfinyl-propionic acid 49639-31-8 C9H10O3S 198.243 —— 3-(benzenesulphonyl)propionyl chloride 3445-53-2 C9H9ClO3S 232.688 3-(苯磺酰)丙腈 3-benzenesulfonyl-propionitrile 10154-75-3 C9H9NO2S 195.242 3-(苯磺酰基)-原丙酸三甲酯 trimethyl 3-(phenylsulfonyl)orthopropionate 38435-08-4 C12H18O5S 274.338 —— benzenesulfonyl-succinic acid —— C10H10O6S 258.252 3-苯硫基丙酸 3-(phenylthio)propionic acid 5219-65-8 C9H10O2S 182.243 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— methyl 3-(phenylsulfonyl)propanoate 10154-72-0 C10H12O4S 228.269 3-(苯基磺酰基)丙酰胺 3-(phenylsulfonyl)propanamide 10154-74-2 C9H11NO3S 213.257 —— 3-(benzenesulphonyl)propionyl chloride 3445-53-2 C9H9ClO3S 232.688 —— 2-methyl-4-(phenylsulfonyl)butan-2-ol 83872-56-4 C11H16O3S 228.312 —— 1-Brom-4-benzolsulfonyl-butanon-(2) 18813-24-6 C10H11BrO3S 291.166 —— [3-(Methoxymethoxy)-3-methylbutyl]sulfonylbenzene 214351-87-8 C13H20O4S 272.365 2-氯乙基苯基砜 2-chloroethyl phenyl sulfone 938-09-0 C8H9ClO2S 204.677 —— N-phenyl-3-(phenylsulfonyl)propanamide 93472-54-9 C15H15NO3S 289.355 —— 4-methyl-1-(2-(phenylsulfonyl)ethyl)-2,6,7-trioxabicyclo[2.2.2]octane 1394135-62-6 C14H18O5S 298.36 N-(4-甲基苯基)-3-(苯基磺酰基)丙酰胺 N-(4-methylphenyl)-3-(phenylsulfonyl)propanamide 340011-42-9 C16H17NO3S 303.382 N-(4-氟苯基)-3-(苯基磺酰基)丙酰胺 N-(4-fluorophenyl)-3-(phenylsulfonyl)propanamide 340011-44-1 C15H14FNO3S 307.345 —— N-(4-bromophenyl)-3-(phenylsulfonyl)propanamide 340011-46-3 C15H14BrNO3S 368.251 N-(4-氯苯基)-3-(苯基磺酰基)丙酰胺 N-(4-chlorophenyl)-3-(phenylsulfonyl)propanamide 340011-45-2 C15H14ClNO3S 323.8 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:五氟苯基酯:使用SmI2和D2O作为氘源,用于化学合成α,α-二氘代乙醇的高度化学选择性的乙炔基前体。摘要:我们报道了使用五氟苯基酯作为酮基自由基前体,SmI2作为温和的还原剂以及D2O作为氘源的氘(≥98%[D2])的精细掺入,首次高度化学选择性地合成α,α-二氘代乙醇。该系统可耐受多种功能基团,可快速进入有价值的α,α-二氘代醇构建基块。更一般地,该报告介绍了五氟苯基酯,这是迄今为止报道的最具反应性的O-酮基前体。DOI:10.1021/acs.orglett.9b04383
-
作为产物:参考文献:名称:Reactions of Mercaptans with Acrylic and Methacrylic Derivatives摘要:DOI:10.1021/ja01202a024
文献信息
-
[EN] COMPOUNDS THAT INHIBIT MCL-1 PROTEIN<br/>[FR] COMPOSÉS INHIBANT LA PROTÉINE MCL-1申请人:AMGEN INC公开号:WO2018183418A1公开(公告)日:2018-10-04Provided herein are myeloid cell leukemia 1 protein (Mcl-1) inhibitors, methods of their preparation, related pharmaceutical compositions, and methods of using the same. For example, provided herein are compounds of Formula (I), or a stereoisomer thereof; and pharmaceutically acceptable salts thereof and pharmaceutical compositions containing the compounds. The compounds and compositions provided herein may be used, for example, in the treatment of diseases or conditions, such as cancer.
-
Piperidine derivative and use thereof申请人:Shirai Junya公开号:US20080275085A1公开(公告)日:2008-11-06The present invention provides a novel piperidine derivative and a tachykinin receptor antagonist containing same, as well as a compound represented by the formula: wherein R 1 is carbamoylmethyl, methylsulfonylethylcarbonyl and the like; R 2 is methyl or cyclopropyl; R 3 is a hydrogen atom or methyl; R 4 is a chlorine atom or trifluoromethyl; R 5 is a chlorine atom or trifluoromethyl; and a group represented by the formula: is a group represented by the formula: wherein R 6 is a hydrogen atom, methyl, ethyl or isopropyl; R 7 is a hydrogen atom, methyl or a chlorine atom; and R 8 is a hydrogen atom, a fluorine atom, a chlorine atom or methyl; or 3-methylthiophen-2-yl, and a salt thereof.
-
Neighboring Group Participation in the Halogenation of Sulfoxides作者:T. Durst、K.-C. Tin、M. J. V. MarcilDOI:10.1139/v73-256日期:1973.6.1
PhS(O)(CH2)nCH2OH(n = 1–3) react with sulfuryl chloride at −78° or 0° in methylene chloride to give the corresponding PhSO2(CH2)nCH2Cl. Evidence is presented that cyclic alkoxyoxosulfonium salts are intermediates in these transformations. When n = 4 only chlorination α to the sulfoxide function was observed. The sulfinyl carboxylic acids PhS(O)(CH2)nCO2H (n = 1–3) and amides PhS(O)(CH2)nCONH2 (n = 1–3) were also reacted with SO2Cl2. α-Chlorination was observed for both compounds when n = 1 or 3. When n = 2 the products were the 3-phenylsulfonylpropionyl chloride and 3-phenyl-sulfonylpropionitrile respectively.
-
Electroreductive Carbofunctionalization of Alkenes with Alkyl Bromides via a Radical-Polar Crossover Mechanism作者:Wen Zhang、Song LinDOI:10.1021/jacs.0c08532日期:2020.12.9variety of alkene functionalization reactions, most of which proceed via an anodic oxidation pathway. In this report, we further expand the scope of electrochemistry to the reductive functionalization of alkenes. In particular, the strategic choice of reagents and reaction conditions enabled a radical-polar crossover pathway wherein two distinct electrophiles can be added across an alkene in a highly电化学允许以受控方式直接访问反应中间体(自由基和离子),以实现选择性有机转化。这一特征已在各种烯烃官能化反应中得到证实,其中大部分是通过阳极氧化途径进行的。在本报告中,我们将电化学的范围进一步扩展到烯烃的还原功能化。特别是,试剂和反应条件的战略选择实现了自由基-极性交叉途径,其中可以以高度化学和区域选择性的方式将两种不同的亲电子试剂添加到烯烃上。具体来说,我们在分子间碳甲酰化、抗马尔科夫尼科夫加氢烷基化、烯烃的羧基化反应——文献中罕见的先例——通过电还原生成烷基自由基和碳负离子中间体。这些反应使用容易获得的起始材料(烷基卤化物、烯烃等)和简单、无过渡金属的条件,并显示出广泛的底物范围和良好的官能团耐受性。通过简单地改变反应介质,可以使用统一的方案来实现所有三种转化。这一发展为构建 Csp3-Csp3 键提供了新的途径。通过简单地改变反应介质,可以使用统一的方案来实现所有三种转化。这一发展为构建
-
Deep Eutectic Solvents as Reaction Media for the Palladium-Catalysed C−S Bond Formation: Scope and Mechanistic Studies作者:Xavier Marset、Gabriela Guillena、Diego J. RamónDOI:10.1002/chem.201702892日期:2017.8.4system based on deep eutectic solvents and palladium nanoparticles where C−S bonds are formed from aryl boronic acids and sodium metabisulfite, is introduced. The functionalization step is compatible with a broad spectrum of reagents such as nucleophiles, electrophiles or radical scavengers. This versatile approach allows the formation of different types of products in an environmentally friendly medium
表征谱图
-
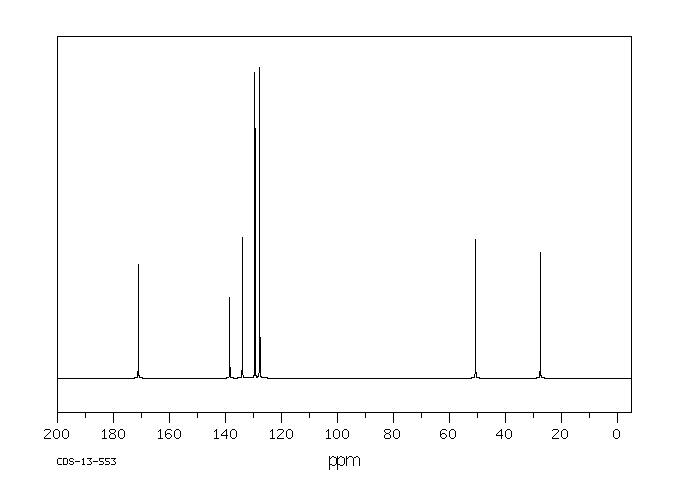
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
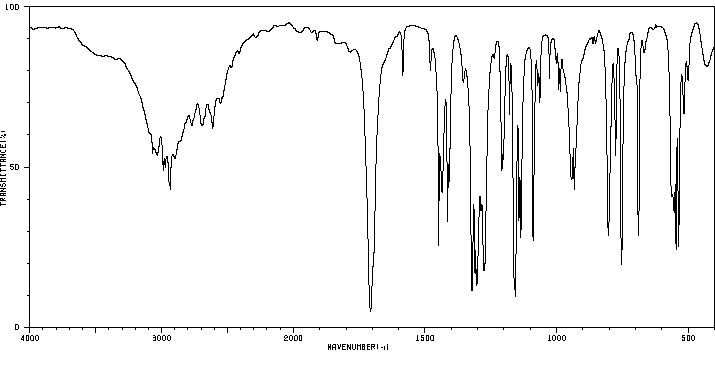
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫