4-硝基苯辛醚 | 49562-76-7
物质功能分类
中文名称
4-硝基苯辛醚
中文别名
对-辛氧基硝基苯
英文名称
1-nitro-4-octyloxybenzene
英文别名
4-octyloxynitrobenzene;4-octyloxy-1-nitrobenzene;p-octaoxynitrobenzene;1-Nitro-4-(octyloxy)benzene;1-nitro-4-octoxybenzene
CAS
49562-76-7
化学式
C14H21NO3
mdl
MFCD00043611
分子量
251.326
InChiKey
WTTNDGCMXADGCJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:225-227 °C (20 mmHg)
-
密度:1.0696 (rough estimate)
-
闪点:38 °C
-
溶解度:可溶于氯仿(少许)、甲醇(少许)
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会发生分解,目前尚未发现有任何已知的危险反应。
计算性质
-
辛醇/水分配系数(LogP):5.3
-
重原子数:18
-
可旋转键数:8
-
环数:1.0
-
sp3杂化的碳原子比例:0.571
-
拓扑面积:55
-
氢给体数:0
-
氢受体数:3
安全信息
-
安全说明:S16
-
危险类别码:R10
-
海关编码:2909309090
-
储存条件:保持贮藏器密封,储存在阴凉、干燥处,并确保工作间有良好的通风或排气装置。
SDS
| Name: | (4-Nitrophenyl)Octyl Ether 99% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 49562-76-7 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 49562-76-7 | (4-Nitrophenyl)Octyl Ether | 99% | unlisted |
Risk Phrases: 10
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
May cause skin irritation. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated. Ingestion of large amounts may cause CNS depression.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation.
May cause burning sensation in the chest.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Water may be ineffective. Use water spray, dry chemical, carbon dioxide, or chemical foam. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Take precautionary measures against static discharges. Keep container tightly closed. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from sources of ignition. Store in a cool, dry place.
Store in a tightly closed container. Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 49562-76-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless to yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 225 - 227 deg C @ 20mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 38 deg C ( 100.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C14H21NO3
Molecular Weight: 251.32
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not currently available.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 49562-76-7 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
(4-Nitrophenyl)Octyl Ether - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: ETHERS, N.O.S. (4-NITROPHENYL)OCTYL ETHER
Hazard Class: 3
UN Number: 3271
Packing Group: III
IMO
Shipping Name: ETHERS, N.O.S. (4-NITROPHENYL)OCTYL ETHER
Hazard Class: 3
UN Number: 3271
Packing Group: III
RID/ADR
Shipping Name: ETHERS, N.O.S. (4-NITROPHENYL)OCTYL ETHER
Hazard Class: 3
UN Number: 3271
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
R 10 Flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 33 Take precautionary measures against static
discharges.
WGK (Water Danger/Protection)
CAS# 49562-76-7: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 49562-76-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 49562-76-7 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对硝基苯酚 4-nitro-phenol 100-02-7 C6H5NO3 139.111 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-辛氧基苯胺 4-octyloxyaniline 39905-45-8 C14H23NO 221.343 —— 4-nitro-N,N-bis(4-(octyloxy)phenyl)aniline 1256087-68-9 C34H46N2O4 546.75 (4-辛氧基-苯基)-胍 (4-octyloxy-phenyl)-guanidine 103714-11-0 C15H25N3O 263.383 —— 4.4'-dioctyloxy-seqcis-azoxybenzene 103468-63-9 C28H42N2O3 454.653 —— 1-<2-Chlormethyl-4-nitro-phenoxy>-octan 5806-70-2 C15H22ClNO3 299.798
反应信息
-
作为反应物:描述:参考文献:名称:血吸虫病的化学疗法。第七部分 2-甲基和2-(取代的甲基)-4-氨基苯基醚摘要:的氯甲基化p硝基苯酚或它的烷基醚鸣哨已被转换成2-羟基甲基- (VII),2- mercaptomethyl-(II),或2-氨基-4-硝基苯基醚(X)的反应性2-氯中间体和各种酰基,烷基,芳基和砜衍生物。已经研究了还原时获得的胺(VIII)作为杀血吸虫剂。DOI:10.1039/j39660000873
-
作为产物:描述:参考文献:名称:CRISTEA, M. F.;JACOB, N.;JACOB, M.;HODOSAN, F. P.摘要:DOI:
文献信息
-
Reversible Formation of a Light‐Responsive Catalyst by Utilizing Intermolecular Cooperative Effects作者:Chloe Z.‐J. Ren、Pablo Solís Muñana、Julien Dupont、Silvia Siru Zhou、Jack L.‐Y. ChenDOI:10.1002/anie.201907078日期:2019.10.21A photoresponsive system where structure formation is coupled to catalytic activity is presented. The observed catalytic activity is reliant on intermolecular cooperative effects that are present when amphiphiles assemble into vesicular structures. Photoresponsive units within the amphiphilic pre-catalysts allow for switching between assembled and disassembled states, thereby modulating the catalytic提出了一种光响应系统,其中结构形成与催化活性偶联。所观察到的催化活性依赖于两亲物组装成囊状结构时存在的分子间协同作用。两亲性预催化剂中的光响应单元允许在组装状态和分解状态之间切换,从而调节催化活性。在动态自组装系统中可逆地形成协同催化剂的能力代表了一种概念上新的工具,可用于设计水中的复杂人工系统。
-
[EN] CALIXPYRROLE COMPOUNDS AND CREATININE-SELECTIVE ELECTRODES COMPRISING THEM<br/>[FR] COMPOSÉS CALIXPYRROLE ET ÉLECTRODES SÉLECTIVES DE CRÉATININE LES COMPRENANT申请人:FUNDACIÓ INST CATALÀ D INVESTIGACIÓ QUÍMICA ICIQ公开号:WO2016116175A1公开(公告)日:2016-07-28These compounds are of formula (la), (lb), (Ic), or are stereoisomers thereof, wherein: R1 is hydrogen, (C1-C20)alkyl; (C3-C20)alkenyl; (C3-C20)alkynyl; (C1- C6)alkyl-O-; (C3-C20)cycloalkyl; (C1-C20)haloalkyl; (C6-C20)aryl optionally substituted; (C6-C20)heteroaryl optionally substituted;; R2 and R2' are hydrogen; (C1-C20)alkyl; (C1-C6)alkyl-O-; (C1-C6)haloalkyl; halogen;cyano; and nitro; to Z1 to Z4 are diradicals of formula (III) wherein A1 and A2 are -O- or -NR3-, wherein R3 is selected from the group consisting of hydrogen and (C1- C20)alkyl; and G is (C1-C6)alkyl;;-P(=S)R5-; -P(=O)R4; -P(=O)(OR4)-; - P(=O)(NR6R7)-; -S(=O)2-; -S(=O)-; or -C(=O)-; and Y1 to Y4 are (C1-C8)alkyl; (C3-C7)cycloalkyl; (C6-C20)aryl optionally substituted; or (C6-C20)heteroaryl optionally substituted; and FG1 and FG2 are H, OH, or NHR8. These compounds are useful as ionophores for creatinine quantification.这些化合物的化学式为(la),(lb),(Ic),或它们的立体异构体,其中:R1为氢,(C1-C20)烷基;(C3-C20)烯基;(C3-C20)炔基;(C1-C6)烷氧基;(C3-C20)环烷基;(C1-C20)卤代烷基;(C6-C20)芳基,可选择性取代;(C6-C20)杂环芳基,可选择性取代;R2和R2'为氢;(C1-C20)烷基;(C1-C6)烷氧基;(C1-C6)卤代烷基;卤素;氰基;和硝基;Z1至Z4为化学式(III)的二自由基,其中A1和A2为-O-或-NR3-,其中R3从氢和(C1-C20)烷基组成;G为(C1-C6)烷基;-P(=S)R5-;-P(=O)R4;-P(=O)(OR4)-;-P(=O)(NR6R7)-;-S(=O)2-;-S(=O)-;或-C(=O)-;Y1至Y4为(C1-C8)烷基;(C3-C7)环烷基;(C6-C20)芳基,可选择性取代;或(C6-C20)杂环芳基,可选择性取代;FG1和FG2为H,OH,或NHR8。这些化合物可用作肌酐定量的离子载体。
-
Light‐Promoted C–N Coupling of Aryl Halides with Nitroarenes作者:Gang Li、Liu Yang、Jian‐Jun Liu、Wei Zhang、Rui Cao、Chao Wang、Zunting Zhang、Jianliang Xiao、Dong XueDOI:10.1002/anie.202012877日期:2021.3A photochemical C–N coupling of aryl halides with nitroarenes is demonstrated for the first time. Catalyzed by a NiII complex in the absence of any external photosensitizer, readily available nitroarenes undergo coupling with a variety of aryl halides, providing a step‐economic extension to the widely used Buchwald–Hartwig C–N coupling reaction. The method tolerates coupling partners with steric‐congestion
-
Aryliminodimagnesium Reagents. XV. Condensation with Nitrobenzene. Formation of Unsymmetrical Azobenzenes Favored by Long-Chain<i>p</i>-Alkoxy-Substituted Reagents作者:Masao Okubo、Koji Matsuo、Akira YamauchiDOI:10.1246/bcsj.62.915日期:1989.3In the reaction of p-MeC6H4N(MgBr)2 with p-alkoxynitrobenzene, no effect of alkoxyl chain length on the relative yields of azoxy and azo products was observed. In contrast, in the reaction of p-alkoxy-C6H4N(MgBr)2 with p-nitrotoluene, C12- and C18-alkoxyl chains led to the corresponding unsymmetrical azobenzenes in unexpectedly high yields. This result, arising from efficient condensation and deoxygenation processes, was explained in terms of cooperation of reagent molecules through their aggregation assisted by a novel tying effect of their long chains.
-
Synthesis of Alkyl-Aryl Ethers by Copper-catalyzed Etherization Reactions of Aryl Fluorides with Tetraalkylammonium Bromides and H2O作者:Feng Wang、Boxiao Tang、Yexiang Xie、Jinheng LiDOI:10.1002/cjoc.201090383日期:2010.11Synthesis of alkyl aryl ethers via copper‐catalyzed etherizations of electron‐deficient aryl fluorides with quaternary ammonium bromides and water has been developed. In the presence of Cu(OAc)2, POPh3 (L4) and Cs2CO3, a variety of electron‐deficient aryl fluorides underwent the reaction with quaternary ammonium bromides and H2O in moderate to good yields. The mechanism was also discussed.
表征谱图
-
氢谱1HNMR
-
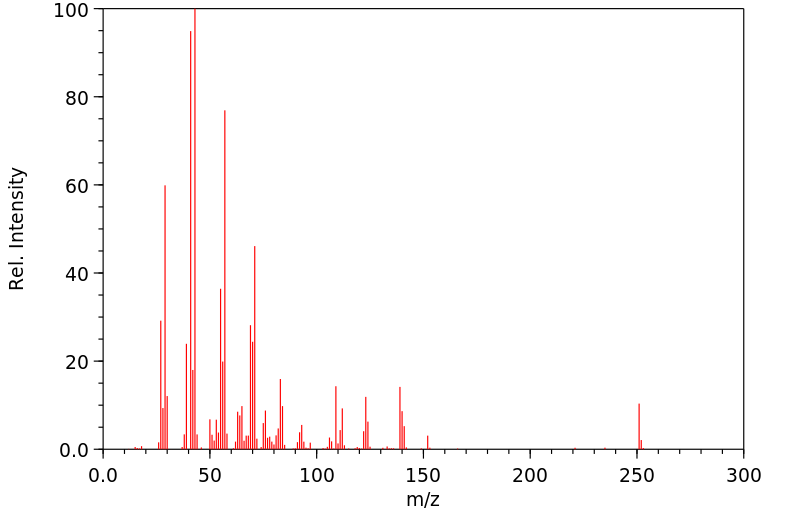
质谱MS
-
碳谱13CNMR
-
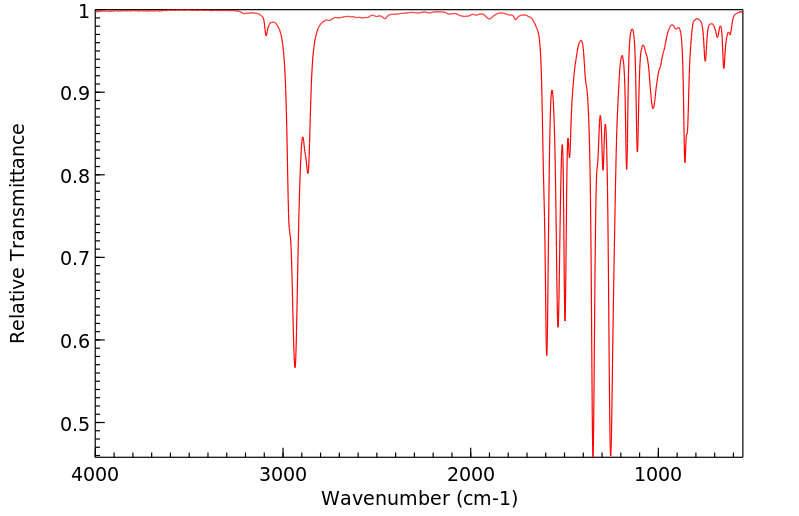
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫