5-硝基吲哚-3-甲醛 | 6625-96-3
中文名称
5-硝基吲哚-3-甲醛
中文别名
5-硝基-1H-吲哚-3-甲醛
英文名称
5-nitro-1H-indole-3-carbaldehyde
英文别名
5-nitroindole-3-carbaldehyde;5-nitroindole-3-carboxaldehyde;5-Nitroindol-3-aldehyd;3-formyl-5-nitroindole;5-nitro-1H-indole-3-carboxaldehyde;5-nitro-3-carboxaldehydeindole;5-Nitroindol-3-carbaldehyd;5-nitro-3-formylindol
CAS
6625-96-3
化学式
C9H6N2O3
mdl
MFCD01313781
分子量
190.158
InChiKey
PHKYMSLVWLYDKP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:300 °C
-
沸点:441.5±25.0 °C(Predicted)
-
密度:1.516±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):1.5
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:78.7
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S24/25,S26,S36/37/39
-
危险类别码:R36/37/38
-
海关编码:2933990090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存放于惰性气体中,避免与空气接触。
SDS
| Name: | 5-Nitro-1h-indole-3-carbaldehyde, 97% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 6625-96-3 |
Synonym:
SECTION 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6625-96-3 | 5-Nitro-1H-indole-3-carbaldehyde | 97% | unlisted |
Risk Phrases: None Listed.
SECTION 3 - HAZARDS IDENTIFICATION EMERGENCY OVERVIEW Not available. Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation. May be harmful if absorbed through the skin.
Ingestion:
May cause irritation of the digestive tract. May be harmful if swallowed.
Inhalation:
May cause respiratory tract irritation. May be harmful if inhaled.
Chronic:
Not available.
SECTION 4 - FIRST AID MEASURES
Eyes:
Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Get medical aid. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Treat symptomatically and supportively.
SECTION 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
SECTION 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container.
SECTION 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes.
Storage:
Store in a cool, dry place. Store in a tightly closed container. Store under an inert atmosphere.
SECTION 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low. Exposure Limits CAS# 6625-96-3: Personal Protective Equipment
Eyes:
Not available.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
SECTION 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 290 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C9H6N2O3
Molecular Weight: 190
SECTION 10 - STABILITY AND REACTIVITY
Chemical Stability:
Not available.
Conditions to Avoid:
Incompatible materials.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported
SECTION 11 - TOXICOLOGICAL INFORMATION RTECS#: CAS# 6625-96-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
5-Nitro-1H-indole-3-carbaldehyde - Not listed by ACGIH, IARC, or NTP.
SECTION 12 - ECOLOGICAL INFORMATION
SECTION 13 - DISPOSAL CONSIDERATIONS Dispose of in a manner consistent with federal, state, and local regulations.
SECTION 14 - TRANSPORT INFORMATION IATA No information available. IMO No information available. RID/ADR No information available.
SECTION 15 - REGULATORY INFORMATION European/International Regulations European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases: S 24/25 Avoid contact with skin and eyes. WGK (Water Danger/Protection) CAS# 6625-96-3: No information available. Canada None of the chemicals in this product are listed on the DSL/NDSL list. CAS# 6625-96-3 is not listed on Canada's Ingredient Disclosure List. US FEDERAL TSCA CAS# 6625-96-3 is not listed on the TSCA inventory. It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
MSDS Creation Date: 6/10/2003 Revision #0 Date: Original. The information above is believed to be accurate and represents the best information currently available to us. However, we make no warranty of merchantability or any other warranty, express or implied, with respect to such information, and we assume no liability resulting from its use. Users should make their own investigations to determine the suitability of the information for their particular purposes. In no way shall the company be liable for any claims, losses, or damages of any third party or for lost profits or any special, indirect, incidental, consequential or exemplary damages, howsoever arising, even if the company has been advised of the possibility of such damages.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
应用
5-硝基吲哚-3-甲醛可用作医药合成中间体。该化合物可通过将5-硝基-1H-吲哚作为反应原料来制备,并用于制备新型的3,5-二取代1H-吲哚衍生物,对胰腺癌细胞株BxPC-3具有较好的抑制活性。
制备
在0℃下,逐步滴加POCl₃(9.2ml,98.5mmol)至DMF(9.5ml,123.1mmol)中,搅拌0.5小时后,加入5-硝基-1H-吲哚(2.0g,12.3mmol),然后在室温下反应1.5小时。随后将反应液倒入大量冰水中,并用6N NaOH调节pH至7,再用乙酸乙酯萃取三次,浓缩后得到2.34g灰色固体5-硝基吲哚-3-甲醛,产率为98%,纯度为95.2%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-硝基芦竹碱 N,N-dimethyl-1-(5-nitro-1H-indol-3-yl)methanamine 3414-64-0 C11H13N3O2 219.243 3-吲哚甲醛 Indole-3-carboxaldehyde 487-89-8 C9H7NO 145.161 5-硝基吲哚 5-nitroindole 6146-52-7 C8H6N2O2 162.148 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-甲基-5-硝基吲哚 3-methyl-5-nitro-1H-indole 61861-88-9 C9H8N2O2 176.175 5-硝基吲哚-3-甲酸 5-nitro-1H-indole-3-carboxylic acid 6958-37-8 C9H6N2O4 206.158 —— 1-methyl-5-nitro-1H-indole-3-carbaldehyde 3558-10-9 C10H8N2O3 204.185 5-硝基吲哚-3-甲腈 5-nitro-1H-indole-3-carbonitrile 7147-14-0 C9H5N3O2 187.158 —— 5-nitroindole-3-methanol 215998-12-2 C9H8N2O3 192.174 5-硝基芦竹碱 N,N-dimethyl-1-(5-nitro-1H-indol-3-yl)methanamine 3414-64-0 C11H13N3O2 219.243 —— (E)-3-(5-nitro-1H-indol-3-yl)acrylonitrile 1363417-40-6 C11H7N3O2 213.195 —— 3-(azidomethyl)-5-nitro-1H-indole 1240467-37-1 C9H7N5O2 217.187 —— bis(5-nitro-1H-indol-3-yl)methane 5031-00-5 C17H12N4O4 336.307 1-苄基-5-硝基-1H-吲哚-3-甲醛 1-benzyl-5-nitroindole-3-carbaldehyde 300664-53-3 C16H12N2O3 280.283 —— (E)-2-methyl-3-(5-nitro-1H-indol-3-yl)acrylic acid 1508307-23-0 C12H10N2O4 246.222 —— 1-methyl-5-nitro-1H-indole-3-carbonitrile —— C10H7N3O2 201.184 5-氨基吲哚-3-甲腈 5-amino-1H-indole-3-carbonitrile 159768-57-7 C9H7N3 157.175 5-硝基吲哚 5-nitroindole 6146-52-7 C8H6N2O2 162.148 —— (E)-ethyl 2-methyl-3-(5-nitro-1H-indol-3-yl)acrylate 1508307-12-7 C14H14N2O4 274.276 —— (E)-3-(5-nitro-1-methyl-1H-indol-3-yl)acrylonitrile 1363417-43-9 C12H9N3O2 227.222 —— ethyl (E)-2-cyano-3-(5-nitro-1H-indol-3-yl)acrylate 1363417-32-6 C14H11N3O4 285.259 5-氨基芦竹碱 3-((dimethylamino)methyl)-1H-indol-5-amine 3414-74-2 C11H15N3 189.26 —— 1,3-dimethyl-5-aminoindole 888216-49-7 C10H12N2 160.219 —— (E)-N-(4-tert-butylphenyl)-2-methyl-3-(5-nitro-1H-indol-3-yl)acrylamide 1508307-37-6 C22H23N3O3 377.443 —— 5-(5-nitro-1H-indol-3-ylmethylene)-pyrimidine-2,4,6-trione 349498-72-2 C13H8N4O5 300.23 —— 6-nitro-γ-carboline 79642-22-1 C11H7N3O2 213.195 —— 5-Amino-3-cyano-1-methylindole 139347-83-4 C10H9N3 171.202 —— 5-((5-nitro-1H-indol-3-yl)methylene)-2,2-dimethyl-1,3-dioxane-4,6-dione 1355714-28-1 C15H12N2O6 316.27 1-Boc-3-羟基甲基-5-硝基吲哚 (N-tert-butyloxycarbonyl-5-nitro-1H-indol-3-yl)methanol 914349-07-8 C14H16N2O5 292.291 —— 5-(5-nitro-1H-indol-3-ylmethylene)-thiazolidine-2,4-dione 503827-04-1 C12H7N3O4S 289.271 —— ethyl (E)-2-cyano-3-(1-methyl-5-nitro-1H-indol-3-yl)acrylate 1363417-36-0 C15H13N3O4 299.286 - 1
- 2
- 3
反应信息
-
作为反应物:描述:5-硝基吲哚-3-甲醛 在 sodium tetrahydroborate 、 Montmorillonite 、 sodium hydride 、 lithium hydroxide 作用下, 以 甲醇 、 乙醇 、 1,2-二氯乙烷 、 乙腈 为溶剂, 反应 4.17h, 生成 3-戊烯-2-酮,3,4-二氟-,(3Z)-(9CI)参考文献:名称:质子交换蒙脱石介导的乙酸异苄酯反应:在扎鲁司特的合成中的应用摘要:具有出色表面特性的质子交换蒙脱石(H-mont)可以在中孔中提供丰富的酸性位点,并且可以作为一种高效的非均相催化剂,用于通过(杂)芳烃的Friedel-Crafts烷基化合成含杂环的二芳基甲烷杂乙酸乙酸酯在温和的反应条件下不需要任何添加剂或惰性气氛。使用该策略,以高收率完成了含吲哚的二芳基甲烷13的克级合成,从而制备了Zafirlukast。此外,H-mont可用于乙酸杂苄酯5p与多种醇和1,3-二羰基化合物的亲核取代反应。DOI:10.1016/j.tetlet.2020.152123
-
作为产物:描述:参考文献:名称:分子碘介导的芳基和杂芳基(二甲基氨基)甲基的C–N键氧化裂解为醛摘要:芳基和杂芳基(二甲基氨基)甲基的C–N键的氧化裂解可通过在环境条件下,弱碱存在下使用分子碘作为弱氧化剂来实现。游离NH和含有各种取代基的取代的吲哚的C 3甲酰化的重要反应是由相应的曼尼希碱完成的。该方法还可以扩展为芳基和其他杂芳基醛和酮的合成。此外,该方法的实用性已成功以克为单位进行了证明。DOI:10.1039/d0nj05832g
文献信息
-
CuI-catalyzed highly regioselective C H functionalization of indoles using indole-3-tosylhydrazones as carbene precursor: An efficient synthesis of 3,3-bis(indolyl)methane derivatives作者:Priya Kamboj、Sunil Dutt、Sourav Chakroborty、Vikas TyagiDOI:10.1016/j.tetlet.2019.151162日期:2019.10Herein, we have developed a novel approach for synthesizing symmetrical and unsymmetrical 3,3-bis(indolyl)methane derivatives via CuI-catalyzed CH functionalization of indole using indole-3-tosylhydrazones as carbene precursor. This procedure works well with different substitutions such as NO2, Br, CH3 and OMe on indole or indole-3-tosylhydrazones and features high regioselectivity for C-3 functionalization
-
Substitution Effect on Near Infrared Absorbance Based Selective Fluoride Sensing of Indole Functionalized Thiourea Molecules作者:Bijit Chowdhury、Sanghamitra Sinha、Pradyut GhoshDOI:10.1002/ejoc.201801534日期:2019.2.7Selective sensing of fluoride within a broad NIR window (825 nm to 1160 nm) by prompt visible color changes by six new symmetrically substituted, thiourea based, indole conjugated sensors is demonstrated. Different substituents lead to specific coloration and absorption spectral shifts in the presence of fluoride. This structure‐property correlation could be helpful to design a series of new NIR based
-
含N-嘧啶吲哚结构的二胺单体及其制备方法
-
Indole derivatives for use as chemical uncoupler
-
[EN] 5-SULFAMOYL-2-HYDROXYBENZAMIDE DERIVATIVES<br/>[FR] DÉRIVÉS DE 5-SULFAMOYL-2-HYDROXYBENZAMIDE申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2017153952A1公开(公告)日:2017-09-14The invention is directed to substituted salicylamide derivatives. Specifically, the invention is directed to compounds according to Formula (I): wherein R, R1 and R2 are as defined herein, or a pharmaceutically acceptable salt thereof. The compounds of the invention are inhibitors of CD73 and can be useful in the treatment of cancer, pre-cancerous syndromes and diseases associated with CD73 inhibition, such as AIDS, the treatment of HIV, autoimmune diseases, infections, atherosclerosis, and ischemia–reperfusion injury. Accordingly, the invention is further directed to pharmaceutical compositions comprising a compound of the invention. The invention is still further directed to methods of inhibiting CD73 activity and treatment of disorders associated therewith using a compound of the invention or a pharmaceutical composition comprising a compound of the invention.
表征谱图
-
氢谱1HNMR
-
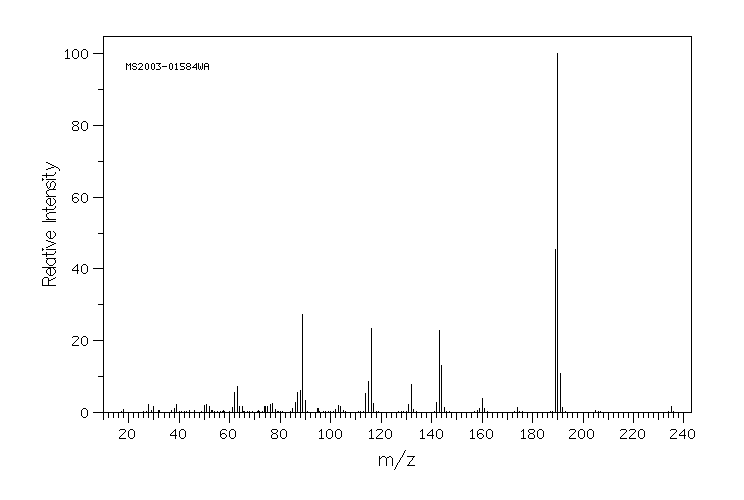
质谱MS
-
碳谱13CNMR
-
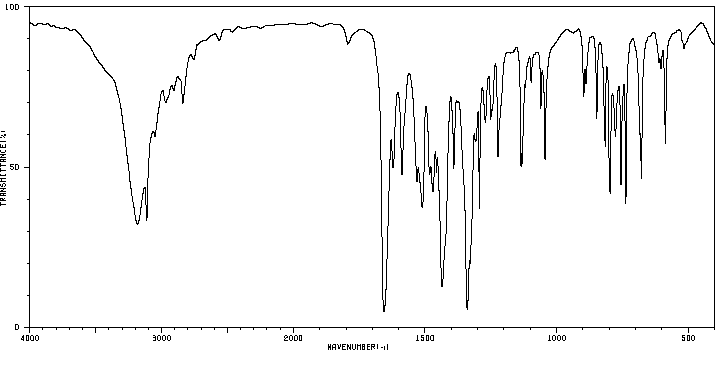
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3