6,6-二甲基二环[3.1.1]庚-2-烯-2-乙醇 | 128-50-7
中文名称
6,6-二甲基二环[3.1.1]庚-2-烯-2-乙醇
中文别名
6,6-二甲基-二环[3.1.1]庚-2-烯-2-乙醇
英文名称
nopol
英文别名
homomyrtenol;2-(6,6-dimethylbicyclo[3.1.1.]hept-2-en-2-yl)-ethan-1-ol;2-(6,6-dimethyl-2-bicyclo[3.1.1]hept-2-enyl)ethanol
CAS
128-50-7
化学式
C11H18O
mdl
MFCD00066419
分子量
166.263
InChiKey
ROKSAUSPJGWCSM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:230-240 °C
-
密度:0.973
-
闪点:98 °C
-
LogP:3.09
-
物理描述:Colroless, slight viscous liquid; mild woody camphoraceous odour
-
溶解度:Insoluble in water; soluble in organic solvents and oils
-
折光率:1.490-1.500
-
保留指数:1194.2
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:12
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:0.818
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xn
-
危险类别码:R22
-
储存条件:密封保存,存放在阴凉干燥处,并远离火源。
SDS
| Name: | Nopol 98% Material Safety Data Sheet |
| Synonym: | 6,6-Dimethylbicyclo[3.1.1]hept-2-ene-2-ethanol; Bicyclo 3.1.1hept-2-ene-2-ethanol, 6,6-dimethyl-; 6,6-Dimethyl-2-norphinene-2-ethanol; 2-Norpinene-2-ethanol, 6,6-dimethyl |
| CAS: | 128-50-7 |
Synonym:6,6-Dimethylbicyclo[3.1.1]hept-2-ene-2-ethanol; Bicyclo 3.1.1hept-2-ene-2-ethanol, 6,6-dimethyl-; 6,6-Dimethyl-2-norphinene-2-ethanol; 2-Norpinene-2-ethanol, 6,6-dimethyl
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 128-50-7 | Nopol | 98 | 204-890-3 |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.
Potential Health Effects
Eye:
Causes eye irritation.
Skin:
Causes moderate skin irritation.
Ingestion:
May be harmful if swallowed. Causes digestive tract irritation.
Inhalation:
Causes respiratory tract irritation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Water may be ineffective. Material is lighter than water and a fire may be spread by the use of water. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Do not ingest or inhale.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 128-50-7: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Viscous liquid
Color: colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 230.0 - 240.0 deg C @ 760.00m
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 98 deg C ( 208.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: .9730g/cm3
Molecular Formula: C11H18O
Molecular Weight: 166.26
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 128-50-7: RC8870000 LD50/LC50:
CAS# 128-50-7: Draize test, rabbit, skin: 500 mg/24H Moderate; Oral, rat: LD50 = 890 mg/kg; Skin, rabbit: LD50 = >5 gm/kg.
Carcinogenicity:
Nopol - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 128-50-7: No information available.
Canada
CAS# 128-50-7 is listed on Canada's DSL List.
CAS# 128-50-7 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 128-50-7 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
制备方法
手性化合物制备材料。
用途简介 用途手性化合物制备材料。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 2-<2-(methoxymethoxy)ethyl>-6,6-dimethylbicyclo<3.1.1>hept-2-ene 77661-66-6 C13H22O2 210.316 —— 2-(6,6-Dimethyl-2-bicyclo[3.1.1]hept-2-enyl)ethoxy-trimethylsilane 69978-56-9 C14H26OSi 238.445 6,6-二甲基-二环[3.1.1]-庚-2-烯-2-乙醇乙酸酯 nopyl acetate 128-51-8 C13H20O2 208.301 (1R)-(-)-诺卜醇苯甲醚 benzyl nopyl ether 74851-17-5 C18H24O 256.388 beta-蒎烯 β-pinene 127-91-3 C10H16 136.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(2-甲氧基乙基)-6,6-二甲基二环[3.1.1]庚-3-烯 2-(2-methoxyethyl)-6,6-dimethylbicyclo<3.1.1>hept-2-ene 81991-72-2 C12H20O 180.29 —— 2-<2-(methoxymethoxy)ethyl>-6,6-dimethylbicyclo<3.1.1>hept-2-ene 77661-66-6 C13H22O2 210.316 —— 2-(6,6-Dimethyl-2-bicyclo[3.1.1]hept-2-enyl)ethoxy-trimethylsilane 69978-56-9 C14H26OSi 238.445 2-[(1R,5S)-6,6-二甲基双环[3.1.1]庚-2-烯-2-基]乙基乙酸酯 nopol acetate 35836-72-7 C13H20O2 208.301 6,6-二甲基-二环[3.1.1]-庚-2-烯-2-乙醇乙酸酯 nopyl acetate 128-51-8 C13H20O2 208.301 —— Nopyl-chlorid 13550-62-4 C11H17Cl 184.709 —— 1-bromo-2-(6,6-dimethylbicyclo[3.1.1.]hept-2-en-2-yl)ethane 55932-64-4 C11H17Br 229.16 6,6-二甲基双环[3.1.1]庚-2-烯-2-乙醛 2-(6,6-Dimethylbicyclo<3.1.1>hept-2-ene)acetaldehyde 30897-75-7 C11H16O 164.247 2-乙烯基-6,6-二甲基双环[3.1.1]庚-2-烯 2-ethylene-6,6-dimethylbicyclo<3.1.1>hept-2-ene 473-00-7 C11H16 148.248 (1R)-(-)-诺卜醇苯甲醚 benzyl nopyl ether 74851-17-5 C18H24O 256.388
反应信息
-
作为反应物:描述:6,6-二甲基二环[3.1.1]庚-2-烯-2-乙醇 在 sodium tetrahydroborate 、 三氟化硼四氢呋喃络合物 、 palladium on activated charcoal 、 氢气 、 sodium hydride 作用下, 以 四氢呋喃 、 乙醇 、 丁酮 为溶剂, 生成 匹维溴铵参考文献:名称:一种匹维溴铵的合成方法摘要:本发明涉及一种匹维溴铵的合成方法,此方法从更加廉价易得的起始原料吗啉和酰化剂入手,合成新的中间体,通过该中间体合成匹维溴铵的反应条件温和,原料反应完全,减少副反应的发生,得到匹维溴铵具有治疗作用的顺式异构体含量≥99%,反式异构体含量≤1%,大幅提高了产物顺式异构体的含量。公开号:CN104650005B
-
作为产物:描述:6,6-二甲基-二环[3.1.1]-庚-2-烯-2-乙醇乙酸酯 在 lithium aluminium tetrahydride 作用下, 以86%的产率得到6,6-二甲基二环[3.1.1]庚-2-烯-2-乙醇参考文献:名称:碳的均质置换:X.甲苯磺酰碘作为甲苯磺酰基自由基的来源,用于形成烯丙基,苄基,环丙基羰基,螺环丙基环烷基,双环[1.0.3]烷基和双环[1.0.4]烷基-4 -有机钴肟的甲苯磺酸酯摘要:4-甲苯磺酰碘在约40°C下与多种烯基和苄基-钴氧肟类发生热反应,可制得高产率的有机-4-甲苯磺酸酯。烯丙基钴肟主要产生,有时在某些情况下仅产生重排的烯砜。脂环族的3-烯基钴肟化合物产生相应的环丙基-羧甲基砜;环烯基乙基钴肟酯产生螺-1,1-环丙基-环烷基砜;环烷-2-烯基甲基钴肟生成双环[1.0。ñ]烷基砜。螺和双环烷基化合物也与其他自由基前体形成。认为反应是通过链机制发生的,其中不定存在或通过底物部分均相形成的钴肟(II)从甲苯磺酰碘中提取碘,生成甲苯磺酰基自由基,该自由基攻击钴肟的有机配体,优选在末端烯烃碳处,从而置换钴肟(II)并得到所观察到的有机产物。DOI:10.1016/0022-328x(85)87417-9
文献信息
-
Reductive amine deallyl- and debenzylation with alkali metal in Silica Gel (M-SG)作者:Partha Nandi、James L. Dye、James E. JacksonDOI:10.1016/j.tetlet.2009.04.058日期:2009.7Alkali metals in silica gel (the M-SG materials) are effective reagents for reductive deallylation, debenzylation, debenzhydrylation, and detritylation of amines. As such, these reagents provide a convenient alternative to traditional metal ammonia solutions for this class of deprotections.
-
Pd‐Colloids‐Catalyzed/Ag <sub>2</sub> O‐Oxidized General and Selective Esterification of Benzylic Alcohols作者:Vaibhav Sable、Jagrut Shah、Anuja Sharma、Anant R. KapdiDOI:10.1002/asia.201900566日期:2019.8Palladium colloids obtained from the degradation of Hermann–Beller palladacycle proved to be an efficient catalytic system in combination with silver oxide as a selective oxidant for the oxidative esterification of differently substituted benzyl alcohols in MeOH as solvent. Excellent reactivity exhibited by the catalytic system also allowed the alcoholic coupling partner to be changed from MeOH to
-
1-(N-Octylthiocarbonyl)-2-(4-thiazolyl)benzimidazole申请人:Merck & Co., Inc.公开号:US04017505A1公开(公告)日:1977-04-12New benzimidazoles substituted at the 1-position with carbonyl substituents and at the 2-position with a 4-thiazolyl group are effective fungicides and anthelmintics. The compounds as well as processes for their preparation are described along with antifungal and anthelmintic compositions for their use. The 1-position substituent is a hydrocarbon group of from 5 to 12 carbon atoms connected to the carbonyl group through an oxygen or a sulfur atom. The compounds are generally prepared by contacting a 1-unsubstituted benzimidazole with a hydrocarbon radical substituted chloroformate or chlorothiol formate.
-
Photochemical transformations. Part XXXII. Photolysis of thiobenzoic acid O-esters. Part III. Photolysis of O-phenethyl thiobenzoates and other thiobenzoates作者:Derek H. R. Barton、Michael Bolton、Philip D. Magnus、Keshav G. Marathe、Gerald A. Poulton、Peter J. WestDOI:10.1039/p19730001574日期:——Homoallylic alcohols can be dehydrated under exceptionally mild conditions by conversion into their thiobenzoate O-esters and irradiation between –70° and room temperature. A variety of substrates have been dehydrated by this method.
-
[EN] S1P RECEPTORS MODULATORS AND THEIR USE THEREOF<br/>[FR] MODULATEURS DES RÉCEPTEURS S1P ET LEUR UTILISATION申请人:AKAAL PHARMA PTY LTD公开号:WO2010043000A1公开(公告)日:2010-04-22The invention relates to novel compounds that have S1P receptor modulating activity. Further, the invention relates to a pharmaceutical comprising at least one compound of the invention for the treatment of diseases and/or conditions caused by or associated with inappropriate S1P receptor modulating activity or expression, for example, autoimmune response. A further aspect of the invention relates to the use of a pharmaceutical comprising at least one compound of the invention for the manufacture of a medicament for the treatment of diseases and/or conditions caused by or associated with inappropriate S1P receptor modulating activity or expression such as autoimmune response.这项发明涉及具有S1P受体调节活性的新化合物。此外,该发明涉及包括本发明中至少一种化合物的药物,用于治疗由不当的S1P受体调节活性或表达引起或相关的疾病和/或病况,例如自身免疫反应。该发明的另一个方面涉及使用包括本发明中至少一种化合物的药物制造药物,用于治疗由不当的S1P受体调节活性或表达引起或相关的疾病和/或病况,如自身免疫反应。
表征谱图
-
氢谱1HNMR
-
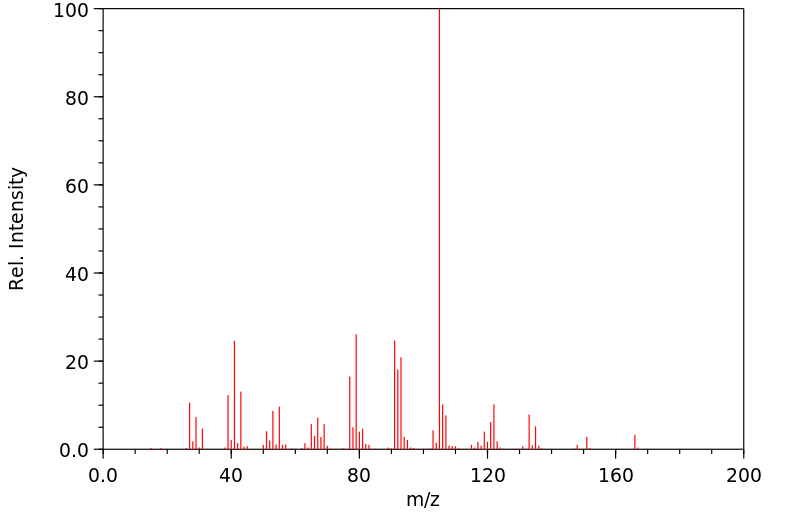
质谱MS
-
碳谱13CNMR
-
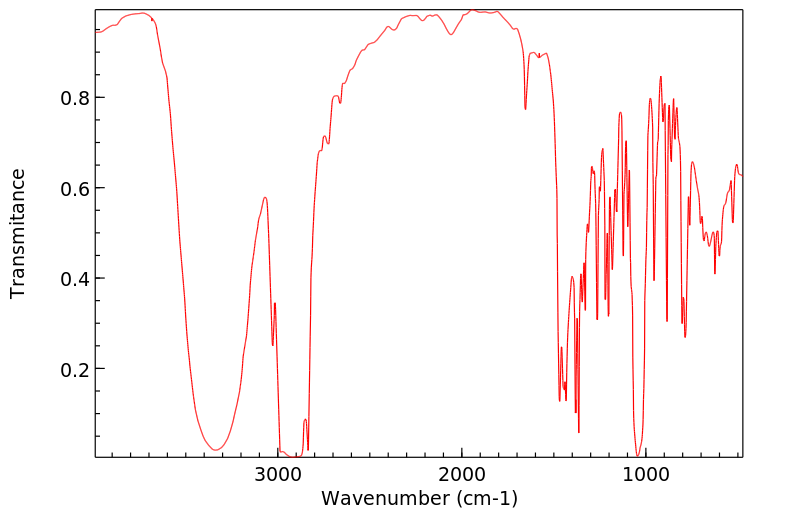
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸