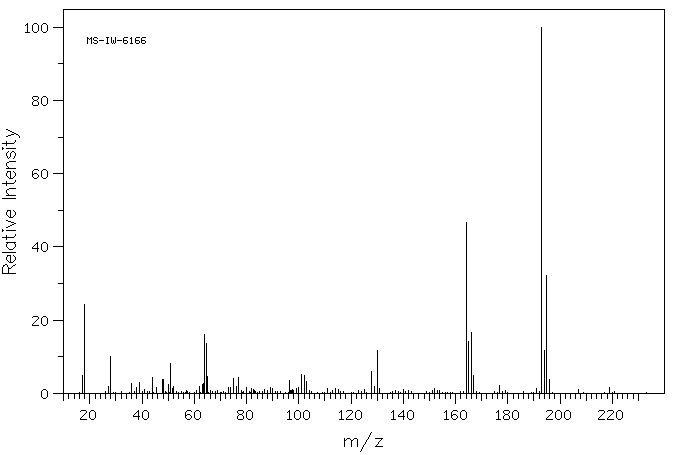
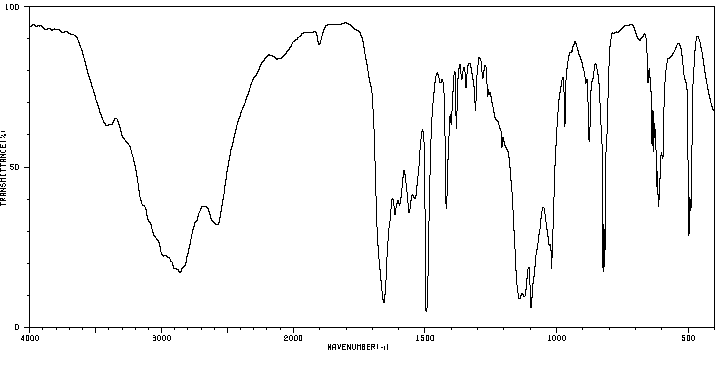
6-氯-2-羟基-4-甲基喹啉 | 2585-04-8
中文名称
6-氯-2-羟基-4-甲基喹啉
中文别名
6-氯-4-甲基喹啉-2-醇;2-羟基-4-甲基-6-氯喹啉
英文名称
4-Methyl-6-chlor-carbostyril
英文别名
6-Chloro-4-methylquinolin-2(1H)-one;6-chloro-4-methyl-1H-quinolin-2-one
CAS
2585-04-8
化学式
C10H8ClNO
mdl
MFCD01001246
分子量
193.633
InChiKey
VQMIYHVFVPSLGB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:IRRITANT
-
海关编码:2933790090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温且干燥环境中保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 6-Chloro-2-hydroxy-4-methylquinoline
Synonyms: 6-Chloro-4-methylquinolin-2(1H)-one
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 6-Chloro-2-hydroxy-4-methylquinoline
CAS number: 2585-04-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H8ClNO
Molecular weight: 193.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 6-Chloro-2-hydroxy-4-methylquinoline
Synonyms: 6-Chloro-4-methylquinolin-2(1H)-one
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 6-Chloro-2-hydroxy-4-methylquinoline
CAS number: 2585-04-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H8ClNO
Molecular weight: 193.6
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-氯-2-氧-1,2-二氢喹啉-4-羧酸 6-chloro-2-hydroxyquinoline-4-carboxylic acid 32431-30-4 C10H6ClNO3 223.616 2,6-二氯-4-甲基喹啉 2,6-Dichlorlepidin 90723-71-0 C10H7Cl2N 212.078
反应信息
-
作为反应物:描述:6-氯-2-羟基-4-甲基喹啉 在 potassium carbonate 、 一水合肼 、 溶剂黄146 、 zinc(II) chloride 作用下, 以 乙醇 、 N,N-二甲基甲酰胺 、 丙酮 为溶剂, 反应 32.0h, 生成 6-chloro-N-[2-(2-chlorophenyl)-4-oxo-1,3-thiazolidin-3-yl]-4-methyl-2-oxoquinoline-1-carboxamide参考文献:名称:Deshmukh; Jadhav; Patil, Journal of the Indian Chemical Society, 1997, vol. 74, # 1, p. 52 - 53摘要:DOI:
-
作为产物:参考文献:名称:HFIP介导的针对β-氧代酰胺的策略,以及随后使用可循环利用的催化剂将Friedel-Craft型环化为2-喹啉酮摘要:以胺和β-酮酸酯为底物,描述了一种简单且经济高效的1,1,1,3,3,3-六氟-2-丙醇(HFIP)介导的合成β-氧代酰胺的方案。发现反应条件对于裂解CO键和由此以优异的产率和选择性形成产物是高度有效的。将所得到的β氧代酰胺在被进一步转化为合成有用2-喹啉酮通过芳族环的分子内弗里德尔-工艺类型环化使用铁氧体作为可回收催化剂。在反应条件下,具有多种官能团的底物的光谱被很好地耐受。文献和质谱证据充分验证了所提出的机制途径。DOI:10.1016/j.tetlet.2020.152535
文献信息
-
Ru‐NHC‐Catalyzed Asymmetric Hydrogenation of 2‐Quinolones to Chiral 3,4‐Dihydro‐2‐Quinolones作者:Tianjiao Hu、Lukas Lückemeier、Constantin Daniliuc、Frank GloriusDOI:10.1002/anie.202108503日期:2021.10.18Direct enantioselective hydrogenation of unsaturated compounds to generate chiral three-dimensional motifs is one of the most straightforward and important approaches in synthetic chemistry. We realized the Ru(II)-NHC-catalyzed asymmetric hydrogenation of 2-quinolones under mild reaction conditions. Alkyl-, aryl- and halogen-substituted optically active dihydro-2-quinolones were obtained in high yields
-
Lactamization of sp<sup>2</sup>C−H Bonds with CO<sub>2</sub>: Transition-Metal-Free and Redox-Neutral作者:Zhen Zhang、Li-Li Liao、Si-Shun Yan、Lei Wang、Yun-Qi He、Jian-Heng Ye、Jing Li、Yong-Gang Zhi、Da-Gang YuDOI:10.1002/anie.201602095日期:2016.6.13The first direct use of carbon dioxide in the lactamization of alkenyl and heteroaryl C−H bonds to synthesize important 2‐quinolinones and polyheterocycles in moderate to excellent yields is reported. Carbon dioxide, a nontoxic, inexpensive, and readily available greenhouse gas, acts as an ideal carbonyl source. Importantly, this transition‐metal‐free and redox‐neutral process is eco‐friendly and desirable
-
Discovery, synthesis and molecular substantiation of N-(benzo[d]thiazol-2-yl)-2-hydroxyquinoline-4-carboxamides as anticancer agents作者:B. Bindu、S. Vijayalakshmi、A. ManikandanDOI:10.1016/j.bioorg.2019.103171日期:2019.10compounds obtained through a four-step synthetic route using a range of substituted acetoacetanilides. Achieved N-(benzo[d]thiazol-2-yl)-2-hydroxyquinoline-4-carboxamides (6a-l) were produced up to 96% of yield while the eco-friendly p-TSA used as a catalyst. Further, the anticancer activity of these compounds was determined using a range of cancer cell lines starting from MCF-7 (Breast cancer), HCT-116努力开发了一系列苯并噻唑和喹啉稠合的生物活性化合物,这些生物活性化合物是通过使用一系列取代的乙酰乙酰苯胺通过四步合成路线而获得的。获得N-(苯并[ d ]噻唑-2-基)-2-羟基喹啉-4-羧酰胺(6a-1)的收率高达96%,而环保型p-TSA用作催化剂。此外,使用一系列癌细胞系(从MCF-7(乳腺癌),HCT-116(结肠癌),PC-3和LNCaP(前列腺)和SK-HEP-1(肝癌)。由于抗氧物质(ROS)对癌症的发展至关重要,因此在抗癌研究之前,也对本研究化合物的抗氧化性能进行了验证。为了确定所述化合物的细胞膜稳定性作用,确定了基于人红细胞(HRBC)的膜保护测定法。结果为化合物6a-1与本研究中使用的其他细胞系相比,它们能够在PC3细胞系(前列腺癌)上产生主要的结果值。由于已知人类生殖细胞碱性磷酸酶(hGC-ALP)在前列腺癌发展中的连通性,因此评估了活性最高的化合物对hGC-ALP的
-
Computational and Synthetic approach with Biological Evaluation of Substituted Quinoline derivatives as small molecule L858R/T790M/C797S triple mutant EGFR inhibitors targeting resistance in Non-Small Cell Lung Cancer (NSCLC)作者:Kshipra S. Karnik、Aniket P. Sarkate、Shailee V. Tiwari、Rajaram Azad、Prasad V.L.S. Burra、Pravin S. WakteDOI:10.1016/j.bioorg.2020.104612日期:2021.2carried out on two different types of EGFR enzymes which include wild-type (PDB: 4I23) and T790M mutated (PDB: 2JIV) respectively. Compounds were also validated upon T790M/C797S mutated (PDB ID: 5D41) EGFR enzyme at the allosteric binding site. All docking studies confirmed high potency and flexibility towards wild type as well as a mutated enzyme. Anticancer activity of the synthesized derivatives通过五步改进的 Suzuki 偶联反应设计和合成了新的取代喹啉衍生物。对两种不同类型的 EGFR 酶进行了比较分子对接研究,分别包括野生型(PDB:4I23)和 T790M 突变型(PDB:2JIV)。还在变构结合位点的 T790M/C797S 突变(PDB ID:5D41)EGFR 酶上验证了化合物。所有对接研究都证实了对野生型以及突变酶的高效力和灵活性。通过标准 MTT 分析检测合成衍生物对 HCC827、H1975(L858R/T790M/C797S 和 L858R/T790M)、A549 和 HT-29 细胞系的抗癌活性。大多数喹啉衍生物显示出显着的细胞毒性作用。发现 4-(4-甲基喹啉-2-基)苯基 4-(氯甲基)苯甲酸酯 (5j) 的 IC50 值为 0。0042 µM、0.02 µM、1.91 µM、3.82 µM 和 3.67 µM,而奥希替尼的 IC50 值分别为 0.0040
-
Free energy perturbation guided Synthesis with Biological Evaluation of Substituted Quinoline derivatives as small molecule L858R/T790M/C797S mutant EGFR inhibitors targeting resistance in Non-Small Cell Lung Cancer (NSCLC)作者:Kshipra S. Karnik、Aniket P. Sarkate、Shailee V. Tiwari、Rajaram Azad、Pravin S. WakteDOI:10.1016/j.bioorg.2021.105226日期:2021.10Two different schemes of novel substituted quinoline derivatives were designed and synthesized via simple reaction steps and conditions. A comparative molecular docking study was carried out on two different types of EGFR enzymes which include wild-type (PDB: 4I23) and T790M mutated (PDB: 2JIV) respectively. Compounds were also validated upon T790M/C797S mutated (PDB ID: 5D41) EGFR enzyme at the allosteric通过简单的反应步骤和条件设计并合成了两种不同的新型取代喹啉衍生物方案。对两种不同类型的 EGFR 酶进行了比较分子对接研究,分别包括野生型(PDB:4I23)和 T790M 突变型(PDB:2JIV)。还在变构结合位点的 T790M/C797S 突变(PDB ID:5D41)EGFR 酶上验证了化合物。进行自由能扰动以确定 ΔG结合形式的蛋白质 - 配体复合物的绝对结合自由能,这反过来提供4ab和5ad通过确定的初始支架中的结构增强成为最有潜力的竞争者。通过标准 MTT 分析检测合成衍生物对 HCC827、H1975 (L858R/T790M)、A549 和 HT-29 细胞系的抗癌活性。化合物4ad (6-chloro-2-( isoindolin -2-yl)-4-methylquinoline) 对突变型 EGFR 激酶显示出优异的抑制活性,IC 50值为 0.91 µM。 通过 insilico
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43