6-羟基-4-甲基香豆素 | 2373-31-1
中文名称
6-羟基-4-甲基香豆素
中文别名
——
英文名称
6-hydroxy-4-methyl-chromen-2-one
英文别名
6-hydroxy-4-methylcoumarin;6-hydroxy-4-methyl-2H-chromen-2-one;6-hydroxy-4-methylchromen-2-one
CAS
2373-31-1
化学式
C10H8O3
mdl
MFCD00016967
分子量
176.172
InChiKey
IRUHWRSITUYICV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:246.5-249.5 °C(lit.)
-
沸点:391.4±37.0 °C(Predicted)
-
密度:1.319±0.06 g/cm3(Predicted)
-
稳定性/保质期:
避免让氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):1.9
-
重原子数:13
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
RTECS号:GN6999800
-
海关编码:2932209090
-
危险性防范说明:P264,P280,P302+P352+P332+P313+P362+P364,P305+P351+P338+P337+P313
-
危险性描述:H315,H319
-
储存条件:储存在阴凉、干燥的地方,并远离热源和不相容物质;需密封保存并在通风良好的环境中存放。
SDS
Section 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE
Product identifiers
Product name : 6-Hydroxy-4-methylcoumarin
CAS-No. : 2373-31-1
Relevant identified uses of the substance or mixture and uses advised against
Identified uses : Laboratory chemicals, Manufacture of substances
Section 2. HAZARDS IDENTIFICATION
Classification of the substance or mixture
Classification according to Regulation (EC) No 1272/2008 [EU-GHS/CLP]
Skin irritation (Category 2)
Eye irritation (Category 2)
Specific target organ toxicity - single exposure (Category 3)
Classification according to EU Directives 67/548/EEC or 1999/45/EC
Irritating to eyes, respiratory system and skin.
Label elements
Labelling according Regulation (EC) No 1272/2008 [CLP]
Pictogram
Signal word Warning
Hazard statement(s)
H315 Causes skin irritation.
H319 Causes serious eye irritation.
H335 May cause respiratory irritation.
Precautionary statement(s)
P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray.
P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove
contact lenses, if present and easy to do. Continue rinsing.
Supplemental Hazard none
Statements
According to European Directive 67/548/EEC as amended.
Hazard symbol(s)
R-phrase(s)
R36/37/38 Irritating to eyes, respiratory system and skin.
S-phrase(s)
S26 In case of contact with eyes, rinse immediately with plenty of water and
seek medical advice.
S36 Wear suitable protective clothing.
Other hazards - none
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substances
Formula : C10H8O3
Molecular Weight : 176,17 g/mol
Component Concentration
6-Hydroxy-4-methyl-2-benzopyrone
CAS-No. 2373-31-1 -
EC-No. 219-146-3
Section 4. FIRST AID MEASURES
Description of first aid measures
General advice
Consult a physician. Show this safety data sheet to the doctor in attendance.
If inhaled
If breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.
In case of skin contact
Wash off with soap and plenty of water. Consult a physician.
In case of eye contact
Rinse thoroughly with plenty of water for at least 15 minutes and consult a physician.
If swallowed
Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.
Most important symptoms and effects, both acute and delayed
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Indication of any immediate medical attention and special treatment needed
no data available
Section 5. FIREFIGHTING MEASURES
Extinguishing media
Suitable extinguishing media
Use water spray, alcohol-resistant foam, dry chemical or carbon dioxide.
Special hazards arising from the substance or mixture
Carbon oxides
Advice for firefighters
Wear self contained breathing apparatus for fire fighting if necessary.
Further information
no data available
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, protective equipment and emergency procedures
Use personal protective equipment. Avoid dust formation. Avoid breathing vapors, mist or gas. Ensure
adequate ventilation. Evacuate personnel to safe areas. Avoid breathing dust.
Environmental precautions
Do not let product enter drains.
Methods and materials for containment and cleaning up
Pick up and arrange disposal without creating dust. Sweep up and shovel. Keep in suitable, closed
containers for disposal.
Reference to other sections
For disposal see section 13.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Avoid contact with skin and eyes. Avoid formation of dust and aerosols.
Provide appropriate exhaust ventilation at places where dust is formed.Normal measures for preventive fire
protection.
Conditions for safe storage, including any incompatibilities
Store in cool place. Keep container tightly closed in a dry and well-ventilated place.
Specific end uses
no data available
Section 8. EXPOSURE CONTROLS/PERSONAL PROTECTION
Control parameters
Components with workplace control parameters
Exposure controls
Appropriate engineering controls
Handle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and
at the end of workday.
Personal protective equipment
Eye/face protection
Safety glasses with side-shields conforming to EN166 Use equipment for eye protection tested
and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).
Skin protection
Handle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique
(without touching glove's outer surface) to avoid skin contact with this product. Dispose of
contaminated gloves after use in accordance with applicable laws and good laboratory practices.
Wash and dry hands.
The selected protective gloves have to satisfy the specifications of EU Directive 89/686/EEC and
the standard EN 374 derived from it.
Body Protection
impervious clothing, The type of protective equipment must be selected according to the
concentration and amount of the dangerous substance at the specific workplace.
Respiratory protection
For nuisance exposures use type P95 (US) or type P1 (EU EN 143) particle respirator.For higher
level protection use type OV/AG/P99 (US) or type ABEK-P2 (EU EN 143) respirator cartridges.
Use respirators and components tested and approved under appropriate government standards
such as NIOSH (US) or CEN (EU).
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Information on basic physical and chemical properties
a) Appearance Form: solid
b) Odour no data available
c) Odour Threshold no data available
d) pH no data available
e) Melting point/freezing Melting point/range: 246,5 - 249,5 °C - lit.
point
f) Initial boiling point and no data available
boiling range
g) Flash point no data available
h) Evaporation rate no data available
i) Flammability (solid, gas) no data available
j) Upper/lower no data available
flammability or
explosive limits
k) Vapour pressure no data available
l) Vapour density no data available
m) Relative density no data available
n) Water solubility no data available
o) Partition coefficient: n- no data available
octanol/water
p) Autoignition no data available
temperature
q) Decomposition no data available
temperature
r) Viscosity no data available
s) Explosive properties no data available
t) Oxidizing properties no data available
Other safety information
no data available
Section 10. STABILITY AND REACTIVITY
Reactivity
no data available
Chemical stability
no data available
Possibility of hazardous reactions
no data available
Conditions to avoid
no data available
Incompatible materials
Strong oxidizing agents
Hazardous decomposition products
Other decomposition products - no data available
Section 11. TOXICOLOGICAL INFORMATION
Information on toxicological effects
Acute toxicity
no data available
Skin corrosion/irritation
no data available
Serious eye damage/eye irritation
no data available
Respiratory or skin sensitization
no data available
Germ cell mutagenicity
no data available
Carcinogenicity
IARC: No component of this product present at levels greater than or equal to 0.1% is identified as
probable, possible or confirmed human carcinogen by IARC.
Reproductive toxicity
no data available
Specific target organ toxicity - single exposure
Inhalation - May cause respiratory irritation.
Specific target organ toxicity - repeated exposure
no data available
Aspiration hazard
no data available
Potential health effects
Inhalation May be harmful if inhaled. Causes respiratory tract irritation.
Ingestion May be harmful if swallowed.
Skin May be harmful if absorbed through skin. Causes skin irritation.
Eyes Causes serious eye irritation.
Signs and Symptoms of Exposure
To the best of our knowledge, the chemical, physical, and toxicological properties have not been
thoroughly investigated.
Additional Information
RTECS: Not available
Section 12. ECOLOGICAL INFORMATION
Toxicity
no data available
Persistence and degradability
no data available
Bioaccumulative potential
no data available
Mobility in soil
no data available
Results of PBT and vPvB assessment
no data available
Other adverse effects
no data available
Section 13. DISPOSAL CONSIDERATIONS
Waste treatment methods
Product
Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed
professional waste disposal service to dispose of this material. Dissolve or mix the material with a
combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber.
Contaminated packaging
Dispose of as unused product.
Section 14. TRANSPORT INFORMATION
UN number
ADR/RID: - IMDG: - IATA: -
UN proper shipping name
ADR/RID: Not dangerous goods
IMDG: Not dangerous goods
IATA: Not dangerous goods
Transport hazard class(es)
ADR/RID: - IMDG: - IATA: -
Packaging group
ADR/RID: - IMDG: - IATA: -
Environmental hazards
ADR/RID: no IMDG Marine pollutant: no IATA: no
Special precautions for user
no data available
Section 15. REGULATORY INFORMATION
This safety datasheet complies with the requirements of Regulation (EC) No. 1907/2006.
Safety, health and environmental regulations/legislation specific for the substance or mixture
no data available
Chemical Safety Assessment
no data available
Section 16. OTHER INFORMATION
Further information
Copyright 2012 Co. LLC. License granted to make unlimited paper copies for internal use
only.
The above information is believed to be correct but does not purport to be all inclusive and shall be
used only as a guide. The information in this document is based on the present state of our knowledge
and is applicable to the product with regard to appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Corporation and its Affiliates shall not be held
liable for any damage resulting from handling or from contact with the above product. See
and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.
制备方法与用途
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基-4-甲基香豆素 6-methoxy-4-methyl-chromen-2-one 6295-35-8 C11H10O3 190.199 6-烯丙氧基-4-甲基-色烯-2-酮 4-methyl-6-allyloxycoumarin 90207-15-1 C13H12O3 216.236 4-甲基香豆素 4-methyl-2H-chromen-2-one 607-71-6 C10H8O2 160.172 —— 2-(6-hydroxy-2-oxo-2H-chromen-4-yl)acetic acid 68747-26-2 C11H8O5 220.182 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基-4-甲基香豆素 6-methoxy-4-methyl-chromen-2-one 6295-35-8 C11H10O3 190.199 —— 6-ethoxy-4-methylcoumarin —— C12H12O3 204.225 —— 6-(2-hydroxyethoxy)-4-methylcoumarin —— C12H12O4 220.225 —— 6-n-propoxy-4-methylcoumarin —— C13H14O3 218.252 —— 4-methyl-6-isopropoxycoumarin —— C13H14O3 218.252 —— 4-methyl-6-(prop-2-ynyloxy)-2H-chromen-2-one —— C13H10O3 214.221 —— 6-hydroxy-2-oxo-2H-chromene-4-carbaldehyde —— C10H6O4 190.155 6-烯丙氧基-4-甲基-色烯-2-酮 4-methyl-6-allyloxycoumarin 90207-15-1 C13H12O3 216.236 —— [(4-Methyl-2-oxo-2H-1-benzopyran-6-yl)oxy]acetonitrile 65031-11-0 C12H9NO3 215.2 —— 4-methyl-6-butoxycoumarin —— C14H16O3 232.279 —— 4-methyl-6-isobutoxycoumarin —— C14H16O3 232.279 —— 4-methyl-6-pentoxycoumarin —— C15H18O3 246.306 —— 4-methyl-6-isopentoxycoumarin —— C15H18O3 246.306 [(4-甲基-2-氧代-2H-苯并吡喃-6-基)氧基]乙酸 2-((4-methyl-2-oxo-2H-chromen-6-yl)oxy)acetic acid 95767-60-5 C12H10O5 234.208 —— 7-hexyloxy-4-methylchromen-2-one —— C16H20O3 260.333 (4-甲基-2-氧代-苯并吡喃-6-基)乙酸酯 acetic acid 4-methyl-2-oxo-2H-chromen-6-yl ester 6345-65-9 C12H10O4 218.209 —— 6-(hex-5-en-1-yloxy)-4-methyl-2H-chromen-2-one —— C16H18O3 258.317 —— 6-(2,3-dihydroxypropoxy)-4-methyl-2H-1-benzopyran-2-one 866240-07-5 C13H14O5 250.251 —— (4-Methyl-2-oxo-2H-chromen-6-yloxy)-acetic Acid Methyl Ester 694508-02-6 C13H12O5 248.235 —— 4-methyl-6-[(oxiran-2-yl)methoxy]-2H-1-benzopyran-2-one 155259-84-0 C13H12O4 232.236 —— 4-methyl-2-oxo-2H-chromen-6-yl acrylate 19090-96-1 C13H10O4 230.22 —— 3,6-dihydroxy-4-methyl-2H-chromen-2-one 1375454-94-6 C10H8O4 192.171 —— ethyl 2-((4-methyl-2-oxo-2H-chromen-6-yl)oxy)acetate 97594-02-0 C14H14O5 262.262 —— 6-ethoxycarbonyloxy-4-methyl-coumarin 354130-60-2 C13H12O5 248.235 —— 6-isobutoxy-2-oxo-2H-chromene-4-carbaldehyde —— C14H14O4 246.26 —— 5,6-dihydroxy-4-methyl-chromen-2-one 5255-59-4 C10H8O4 192.171 —— hexadecanoic acid 4-methyl-2-oxo-2H-chromen-6-yl ester 959582-92-4 C26H38O4 414.585 —— 5-formyl-6-hydroxy-4-methylcoumarin 91136-60-6 C11H8O4 204.182 —— 6-(3,4-dicyanophenoxy)-4-methylcoumarin —— C18H10N2O3 302.289 —— 4-methyl-2-oxo-2H-chromen-6-yl trifluoromethanesulfonate 901482-26-6 C11H7F3O5S 308.235 —— 5-amino-6-hydroxy-4-methyl-coumarin 5255-58-3 C10H9NO3 191.186 —— O,O-diethyl-O-(4-methyl-6-coumarino)-phosphorothioate 109161-68-4 C14H17O5PS 328.326 —— 6-chloro-4-methyl-2H-chromen-2-one 24145-78-6 C10H7ClO2 194.617 —— 4-methyl-7-[4-(4-methylbenzopyran-2-one-7-yloxy)butyloxy]benzopyran-2-one 66346-21-2 C24H22O6 406.435 —— 1,5-Bis-(4'-methyl-coumarin-7'-oxy)-pentan 23863-89-0 C25H24O6 420.462 —— 5,7-Diamino-6-hydroxy-4-methylchromen-2-one 852242-89-8 C10H10N2O3 206.201 —— 6-hydroxy-4-methyl-7-nitro-2H-chromen-2-one —— C10H7NO5 221.169 —— (4-Methyl-2-oxochromen-6-yl) anthracene-9-carboxylate —— C25H16O4 380.4 —— 9-methyl-2H,7H-pyrano<2,3-g><1>benzopyran-7-one —— C13H10O3 214.221 —— 4-methyl-6-(toluene-4-sulfonyloxy)-coumarin 69830-32-6 C17H14O5S 330.361 —— 5,6-dimethoxy-4-methyl-coumarin 91963-62-1 C12H12O4 220.225 —— 6-hydrazinyl-4-methyl-2H-chromen-2-one —— C10H10N2O2 190.202 —— 6-chloro-2-oxo-2H-chromene-4-carbaldehyde —— C10H5ClO3 208.601 —— 4-Methyl-6-[(4-methylidene-5-oxooxolan-2-yl)methoxy]chromen-2-one —— C16H14O5 286.284 —— (E)-6-hydroxy-3-(4-hydroxy-3,5-dimethylstyryl)-4-methyl-2H-chromen-2-one 1456809-28-1 C20H18O4 322.361 —— 6-hydroxy-3-((4-hydroxy-3,5-dimethylphenyl)ethynyl)-4-methyl-2H-chromen-2-one 1456809-33-8 C20H16O4 320.345 —— 5,7-dinitroso-6-hydroxy-4-methylcoumarin 182954-16-1 C10H6N2O5 234.168 —— (E)-3-(4-hydroxy-3,5-dimethylstyryl)-6-methoxy-4-methyl-2H-chromen-2-one 1456809-26-9 C21H20O4 336.387 —— 4-methyl-8,11-dihydro-3H-oxepino[3,2-f]chromen-3-one 1350971-97-9 C14H12O3 228.247 —— 1-(4'-methylcoumarin-6'-oxy)-3-(3''-methylbenzisoxazol-6''-oxy)-2-hydroxypropane —— C21H19NO6 381.385 —— (E)-3-(4-hydroxy-3,5-dimethylstyryl)-7-methoxy-4-methyl-2H-chromen-2-one 1456809-31-6 C21H18O4 334.372 —— 6-hydroxy-4-methyl-5-nitro-2H-chromen-2-one 5255-54-9 C10H7NO5 221.169 —— 7-chloro-4-[(4-methyl-2-oxo-2H-chromen-6-yloxy)methyl]quinolin-2(1H)-one 1299366-37-2 C20H14ClNO4 367.788 —— 6-chloro-4-[(4-methyl-2-oxo-2H-chromen-6-yloxy)methyl]quinolin-2(1H)-one 1299366-36-1 C20H14ClNO4 367.788 —— 8-methyl-4-[(4-methyl-2-oxo-2H-chromen-6-yloxy)methyl]quinolin-2(1H)-one 1299366-39-4 C21H17NO4 347.37 —— 4-methylumbelliferyl β-D-glucopyranoside —— C16H18O8 338.314 —— 5-Acetyl-6-(acetyloxy)-4-methyl-2H-1-benzopyran-2-one 129812-33-5 C14H12O5 260.246 —— 6-hydroxy-4-methyl-5,7-dinitrocoumarin 102589-06-0 C10H6N2O7 266.167 - 1
- 2
- 3
- 4
- 5
- 6
反应信息
-
作为反应物:描述:参考文献:名称:Microwave-Assisted Synthesis of Novel 2H-Chromene Derivatives Bearing Phenylthiazolidinones and Their Biological Activity Assessment摘要:制备了 6-羟基-2-氧代-2H-苯并吡喃-4-甲醛 (2)、6-氯-2-氧代-2H-苯并吡喃-4-甲醛 (3) 和 6-肼基-4-甲基-2H-苯并吡喃-2-酮 (5),作为单药效基团关键中间体。在微波辅助和传统化学合成工艺下,将化合物 2、3 和 5 加入一系列多组分反应 (MCR),以良好的产率获得了一系列三和/或四药效基团共轭物 8a、b、11、13、16、17、19 和 20。新合成的化合物通过红外光谱、核磁共振、13C-核磁共振、质谱和元素分析进行了表征。最后,对合成的化合物进行了生物活性筛选,结果表明它们对不同种类的细菌和真菌具有显著的抗菌活性。DOI:10.3390/molecules191219648
-
作为产物:描述:6-烯丙氧基-4-甲基-色烯-2-酮 在 palladium on activated charcoal ammonium formate 作用下, 以 甲醇 为溶剂, 反应 1.5h, 以95%的产率得到6-羟基-4-甲基香豆素参考文献:名称:使用钯/炭催化剂和甲酸铵对苯酚和羟基香豆素的烯丙基醚进行温和有效的脱保护摘要:描述了一种催化量为10%的Pd / C与甲酸铵相结合的烯丙基苯基醚和烯丙基香豆基醚裂解的有效方法。酚的烯丙基醚比醇的烯丙基醚优先脱保护,并且该方法与几种可还原的官能团如CHO,COCH 3,CO 2 Et和NHCOCH 3相容。DOI:10.1016/j.tetlet.2006.05.176
文献信息
-
Supramolecular Control of the Template-Induced Selective Photodimerization of 4-Methyl-7-O-hexylcoumarin作者:William G. Skene、Emilie Couzigné、Jean-Marie LehnDOI:10.1002/chem.200305268日期:2003.11.21are capable of binding substrates with complementary donor and acceptor sites to form a supramolecular complex through hydrogen bonding. Receptor 3 was designed to accept two guest molecules, which are held in close proximity within the supramolecular species. The substrate molecule, 4-methyl-7-O-hexylcoumarin (1 c), forms a 2:1 complex with a binding constant of 150 m(-1) for the second substrate设计并合成了包含两个相同的氢键识别亚基的对称的对位分子受体(3)。这些亚基能够结合具有互补供体和受体位点的底物以通过氢键形成超分子复合物。受体3被设计为接受两个客体分子,它们紧密地保持在超分子物种内。底物分子4-甲基-7-O-己基香豆素(1 c)与第二种底物形成2:1配合物,其结合常数为150 m(-1),首先通过1:1配合物与亲和常数510 m(-1)。当1 c的两个分子与模板结合时,其取向会导致在照射时选择性形成反式[2 + 2]光合产物2 cB。
-
신규한 쿠마린 유도체 및 이의 제조방법申请人:GREEN CROSS MEDIS Corp. 주식회사 녹십자메디스(120090236968) Corp. No ▼ 135111-0087407BRN ▼410-81-93457公开号:KR101620093B1公开(公告)日:2016-05-13본 발명은 신규한 쿠마린 유도체 및 이의 제조방법에 관한 것이다. 본 발명에 따른 쿠마린 유도체는 수용액에서 용해도가 우수하고 형광을 갖는 쿠마린 모체에 아미노페닐보론산을 도입함으로써, 당화혈색소와 높은 결합력을 갖는 보론산에 의해 혈액 속에 있는 당화혈색소와 결합력이 높아져 혈액 속의 당화혈색소 존재 유무를 선택적 발광성을 통해 확인할 수 있으며, 당화혈색소의 인지 능력을 증대시킬 수 있다. 따라서, 본 발명에 따른 쿠마린 유도체는 당화혈색소에 대하여 선택적 결합력이 있고 결합 전후에 형광 변화를 나타낼 수 있으므로, 혈액에서 당화혈색소 검출용 센서로 유용하게 사용될 수 있다.
-
Cross-Coupling of Amide and Amide Derivatives to Umbelliferone Nonaflates: Synthesis of Coumarin Derivatives and Fluorescent Materials作者:Shane M. Hickey、Samuel O. Nitschke、Martin J. Sweetman、Christopher J. Sumby、Douglas A. Brooks、Sally E. Plush、Trent D. AshtonDOI:10.1021/acs.joc.0c00813日期:2020.6.19properties of aqueous-soluble derivatives were determined, and they displayed auxochrome-based variations. Gram-scale synthesis provided an acrylamide analogue, which was used to fabricate a fluorescent poly(2-hydroxylethyl methacrylate) (pHEMA) hydrogel that was resistant to leaching in ultrapure H2O. We envisage that our reported protocol to access 7-amino-4-methylcoumarin derivatives will find use toward
-
Quaternary ammonium and amido derivatives of pyranochromenones and chromenones: synthesis and antimicrobial activity evaluation作者:Suchita Prasad、Shiv Kumar、Bipul Kumar、Abhishek Kumar Singh、Hemant K. Gautam、Sunil K. SharmaDOI:10.1007/s00044-014-1294-4日期:2015.6of their resistance to antimicrobial agents are alarming issues worldwide and consistent efforts are being made to develop efficient antimicrobial agents. In this perspective, a series of novel ammonium and amide derivatives of pyranocoumarin and coumarin were synthesized and evaluated for their antimicrobial activity. Among them, six compounds exhibited significant activity against gram-positive bacteria传染性疾病及其对抗菌剂的抗性发展是世界范围内令人震惊的问题,并且人们一直在努力开发有效的抗菌剂。从这个角度出发,合成了一系列新颖的吡喃香豆素和香豆素铵和酰胺衍生物,并对其抗菌活性进行了评估。其中,六种化合物对革兰氏阳性细菌蜡状芽孢杆菌(MTCC 430)和枯草芽孢杆菌(MTCC 121)和革兰氏阴性细菌铜绿假单胞菌(MTCC 741)表现出显着活性。化合物N,N,N-三乙基-10-(4,8,8-三甲基-2-氧代-2,6,7,8-四氢吡喃[3,2 - g ]铬基-10-基氧基)癸1 -溴化铵(16)在MDA中表现出最高的抗菌活性,MIC值为5μg/ ml。化合物17和18对MDA中的红毛癣菌(临床分离株)表现出中等的抗真菌活性,MIC为6.25μg/ ml 。溶血分析结果表明,六分之三的化合物各自的MIC值是安全的。这些结果为进一步优化用于设计下一代化合物作为先导抗菌剂的支架提供了见识。
-
[EN] NOVEL CHROMEN-2-ONE DERIVATIVES AND THEIR USE AS MONOAMINE NEUROTRANSMITTER RE-UPTAKE INHIBITORS<br/>[FR] NOUVEAUX DERIVES DE CHROMENE-2-ONE ET LEUR UTILISATION COMME INHIBITEURS DE RECAPTURE DU NEUROTRANSMETTEUR MONOAMINE申请人:NEUROSEARCH AS公开号:WO2006035034A1公开(公告)日:2006-04-06This invention relates to novel chromen-2-one derivatives useful as monoamine neurotransmitter re-uptake inhibitors. In other aspects the invention relates to the use of these compounds in a method for therapy and to pharmaceutical compositions comprising the compounds of the invention.
表征谱图
-
氢谱1HNMR
-
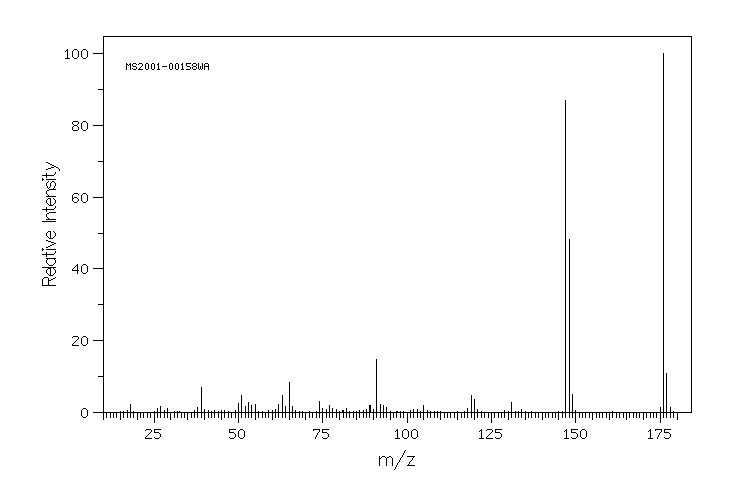
质谱MS
-
碳谱13CNMR
-
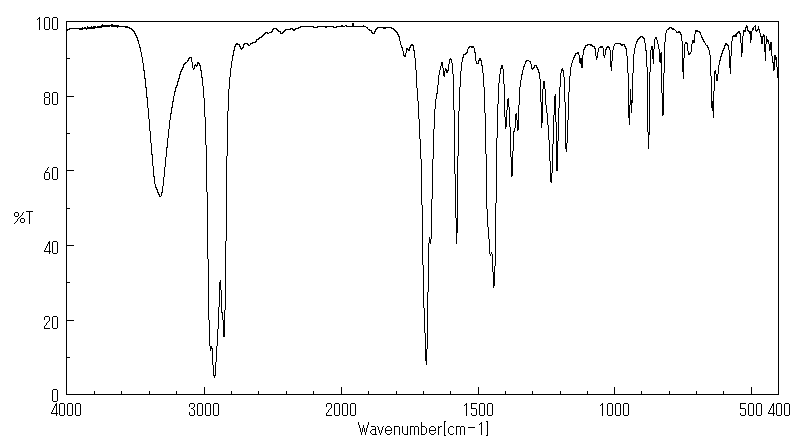
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
黄皮香豆精
黄木亭
黄曲霉素P2
黄曲霉素P1
黄曲霉素G2-13C17-同位素
黄曲霉素G2
黄曲霉素G1-13C17-同位素
黄曲霉素B2-13C17-同位素
黄曲霉素B1-13C17-同位素
黄曲霉素B1 8,9-环氧化物
黄曲霉素 G1
黄曲霉毒醇Ⅱ
黄曲霉毒醇M1
黄曲霉毒醇A
黄曲霉毒素M2
黄曲霉毒素M1-(O-羧甲基)肟
黄曲霉毒素G2a
黄曲霉毒素G19,10-环氧化物
黄曲霉毒素B2
黄曲霉毒素B1二氯化物
黄曲霉毒素B1-8,9-二氯化物
黄曲霉毒素B1-(O-羧甲基)肟
黄曲霉毒素 Q1
黄曲霉毒素 M1
黄曲霉毒素 B2
黄曲霉毒素 B1
黄曲霉毒素
香豆霉素
香豆素6H
香豆素545T
香豆素545
香豆素525
香豆素343甲酯
香豆素338
香豆素314T
香豆素175
香豆素152
香豆素106
香豆素-D4
香豆素-6-磺酰氯
香豆素-6-甲醛
香豆素-5-氧丁酸
香豆素-4-乙酸
香豆素-3腈
香豆素-35
香豆素-3-羧酸酸酐
香豆素-3-羧酸琥珀酰亚胺酯
香豆素-3-羧酸乙酯
香豆素-3-羧酸
香豆素-3-甲酰氯