4-甲酰基-2-甲基苯硼酸 | 156428-81-8
中文名称
4-甲酰基-2-甲基苯硼酸
中文别名
4-甲酰基-2-甲基苯基硼酸
英文名称
(4-formyl-2-methylphenyl)boronic acid
英文别名
4-Formyl-2-methylphenylboronic acid
CAS
156428-81-8
化学式
C8H9BO3
mdl
MFCD09839115
分子量
163.969
InChiKey
ORHODDYPZMCLBU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:140 - 143°C
-
沸点:357.7±52.0 °C(Predicted)
-
密度:1.20±0.1 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):1.78
-
重原子数:12
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:57.5
-
氢给体数:2
-
氢受体数:3
安全信息
-
海关编码:2931900090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-Formyl-2-methylphenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Formyl-2-methylphenylboronic acid
CAS number: 156428-81-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H9BO3
Molecular weight: 164.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-Formyl-2-methylphenylboronic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-Formyl-2-methylphenylboronic acid
CAS number: 156428-81-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H9BO3
Molecular weight: 164.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— [2-methyl-4-(1-pyrrolidinylmethyl)phenyl]boronic acid 1366131-43-2 C12H18BNO2 219.091
反应信息
-
作为反应物:参考文献:名称:[EN] AMINOQUINOLINE DERIVATIVES AS ANTIVIRAL AGENTS
[FR] DÉRIVÉS D'AMINOQUINOLÉINE COMME AGENTS ANTIVIRAUX摘要:提供的是式(I)和式(II)的化合物及其药用盐,它们的药物组合物,它们的制备方法,以及它们用于治疗由黄病毒科病毒介导的病毒感染,如丙型肝炎病毒(HCV)。公开号:WO2012037108A1 -
作为产物:描述:参考文献:名称:EP3736266摘要:公开号:
文献信息
-
[EN] NAPHTHYRIDINE DERIVATIVES AS PRC2 INHIBITORS<br/>[FR] DÉRIVÉS DE NAPHTYRIDINE EN TANT QU'INHIBITEURS DE PRC2申请人:MIRATI THERAPEUTICS INC公开号:WO2020219448A1公开(公告)日:2020-10-29Disclosed are compounds of formula (I) or (II) that inhibit Polycomb Repressive Complex 2 (PRC2) activity. In particular, the present invention relates to compounds, pharmaceutical compositions and methods of use, such as methods of treating cancer using the compounds and pharmaceutical compositions of the present invention.揭示了抑制Polycomb Repressive Complex 2 (PRC2)活性的化合物,其化学式为(I)或(II)。具体来说,本发明涉及化合物、药物组合物和使用方法,例如使用本发明的化合物和药物组合物治疗癌症的方法。
-
Unique Polypharmacology Nuclear Receptor Modulator Blocks Inflammatory Signaling Pathways作者:Mi Ra Chang、Anthony Ciesla、Timothy S. Strutzenberg、Scott J. Novick、Yuanjun He、Ruben D. Garcia-Ordonez、Rebecca L. Frkic、John B. Bruning、Theodore M. Kamenecka、Patrick R. GriffinDOI:10.1021/acschembio.9b00236日期:2019.5.17we show that the pan modulator SR1903 effectively blocks TREM-1 activation. SR1903 emerged from a chemical series of potent RORγ inverse agonists, although unlike close structural analogues, it has modest agonist activity on LXR and weak repressive activity (inverse agonism) of PPARγ, three receptors that play essential roles in inflammation and metabolism. The anti-inflammatory and antidiabetic efficacy肥胖和风湿病通过慢性炎症在机制上相关。孤儿受体 TREM-1(骨髓细胞上表达的触发受体-1)是促炎和非感染性免疫反应的有效放大器。在这里,我们证明泛调节器 SR1903 有效阻止 TREM-1 激活。SR1903 源自一系列强效 RORγ 反向激动剂化学系列,尽管与结构紧密的类似物不同,它对 LXR 具有适度的激动剂活性,而对 PPARγ 具有较弱的抑制活性(反向激动),这三种受体在炎症和代谢中发挥重要作用。这种独特的调节剂在胶原诱导的关节炎和饮食诱导的肥胖小鼠模型中具有抗炎和抗糖尿病功效。有趣的是,在肥胖的背景下,与选择性 RORγ 反向激动剂不同,SR1903 有助于维持胸腺稳态。SR1903 在长期给药后具有良好的耐受性,综合起来,这些数据表明它可能代表了治疗代谢性疾病和炎症性疾病的可行策略。更重要的是,SR1903 阻断 LPS 信号传导的能力表明这种独特的多药理调节剂在治疗先天免疫反应疾病方面的潜在用途。
-
유기염료, 이를 포함하는 조성물 및 염료감응 태양전지申请人:UNIST(ULSAN NATIONAL INSTITUTE OF SCIENCE AND TECHNOLOGY) 울산과학기술원(120150812047) Corp. No ▼ 230171-0011595BRN ▼620-82-06236公开号:KR20200142800A公开(公告)日:2020-12-23본 발명은, 유기염료, 이를 포함하는 조성물 및 염료감응 태양전지에 관한 것으로, 비틀린 π-스페이스기(twisted π-spacer) 및 DPP계 코어 유닛이 도입된, 유기염료, 이를 포함하는 조성물 및 염료감응 태양전지에 관한 것이다.
-
Exploiting Conformational Dynamics To Facilitate Formation and Trapping of Electron-Transfer Photoproducts in Metal Complexes作者:Heather A. Meylemans、Joshua T. Hewitt、Mirvat Abdelhaq、Paul J. Vallett、Niels H. DamrauerDOI:10.1021/ja1055559日期:2010.8.25are reported based on electrochemical measurements of 1-3 as well as Franck-Condon analysis of emission spectra collected for three new donor model complexes 1'-3'. These preserve the substitution patterns on the aryl substituent in their respective D-A complexes but remove the bipyridinium acceptor. Both DeltaG(ET) and DeltaG(BET) are invariant to within 0.02 eV across the series. Upon visible photoexcitation报告了三种新的光致电子供体 - 受体 (DA) 系统,它们通过参与 D 和 A 之间桥梁的构象活性不对称芳基取代联吡啶配体将 Ru(II) 激发态供体与联吡啶受体并置。在配合物 1-3 中,依次添加空间位阻以调节联吡啶和芳基取代基之间的环间二面角θ。光致电子转移 (DeltaG(ET)) 和反电子转移 (DeltaG(BET)) 的驱动力是基于 1-3 的电化学测量以及对三个新供体模型复合物收集的发射光谱的 Franck-Condon 分析报告的 1 '-3'。它们保留了各自 DA 复合物中芳基取代基上的取代模式,但去除了联吡啶鎓受体。DeltaG(ET) 和 DeltaG(BET) 在整个系列中都保持在 0.02 eV 以内。在 500 +/- 10 nm 处使用大约 100 fs 激光脉冲对每个 DA 系统进行可见光激发后,观察到电子转移 (ET) 光产物形成,时间常数为 tau(ET)
-
Molecular design strategy for realizing vectorial electron transfer in photoelectrodes作者:Deok-Ho Roh、Jun-Hyeok Park、Hyun-Gyu Han、Ye-Jin Kim、Daiki Motoyoshi、Eunhye Hwang、Wang-Hyo Kim、Joseph I. Mapley、Keith C. Gordon、Shogo Mori、Oh-Hoon Kwon、Tae-Hyuk KwonDOI:10.1016/j.chempr.2022.01.017日期:2022.4Vectorial electron transfer is essential for developing ideal artificial photosystems. Conventional photosensitizers with only strong electronic coupling, as used in photovoltaics, cannot achieve vectorial electron transfer because of their rapid charge recombination. Herein, we present a design strategy for integrating strong (∼600 cm−1) and relatively weak (310 cm−1) electronic coupling in a single矢量电子转移对于开发理想的人造光系统至关重要。传统的仅具有强电子耦合的光敏剂,如用于光伏发电,由于其快速的电荷复合,无法实现矢量电子转移。在此,我们提出了一种整合强(~600 cm -1)和相对弱(310 cm -1) 单分子中的电子耦合以实现染料敏化太阳能电池中的矢量电子转移。开发了四种具有供体-受体-π-间隔物和锚定基团构型的敏化剂,这些敏化剂中的电子耦合由不同空间位阻的π-间隔物控制。瞬态吸收光谱揭示了设计的敏化剂中矢量电子转移的发生,通过减弱受体和π-间隔物之间的电子耦合抑制电荷复合,以及通过供体和受体之间的强电子耦合实现有效的电荷注入。敏化剂中电子耦合的优化提高了光伏性能,实现了10.8%的功率转换效率。
表征谱图
-
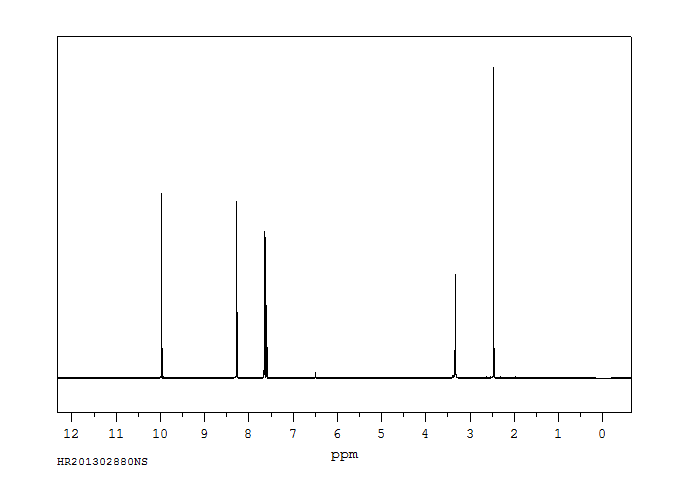
氢谱1HNMR
-
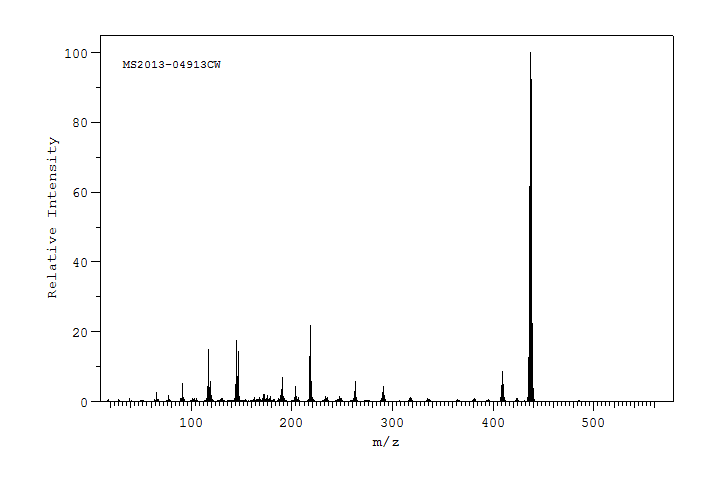
质谱MS
-
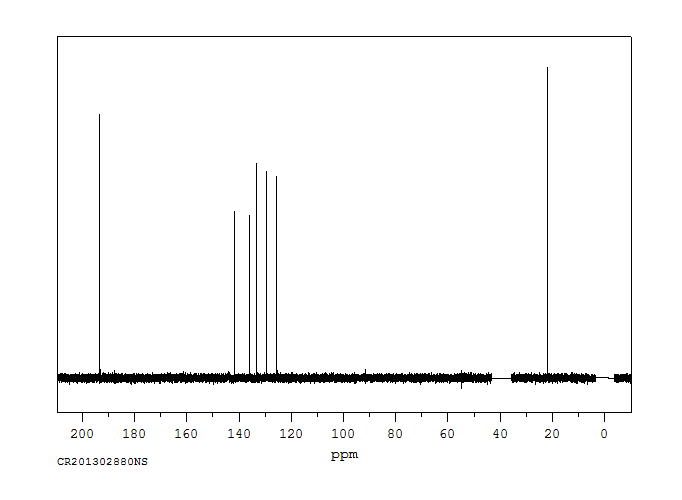
碳谱13CNMR
-
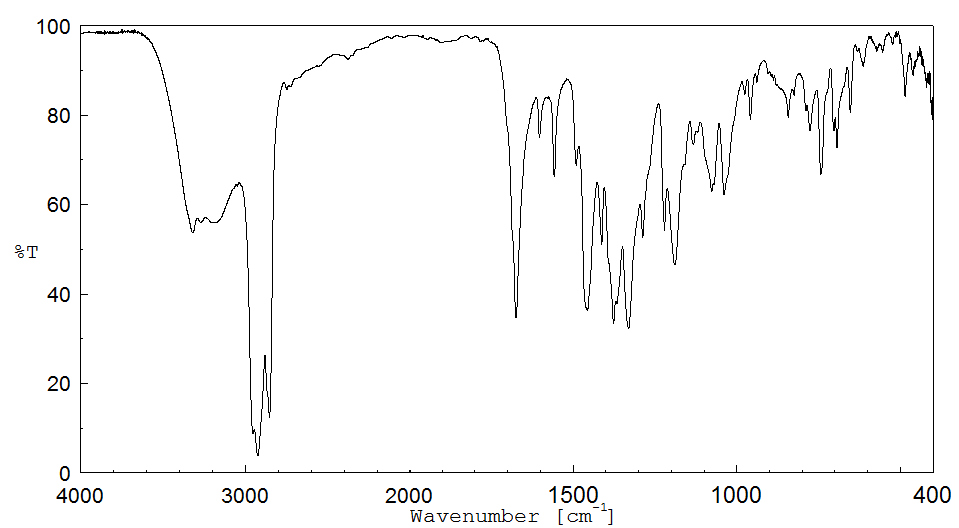
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫