1-丙基膦酸二乙酯 | 18812-51-6
中文名称
1-丙基膦酸二乙酯
中文别名
1-丙基磷酸二乙酯;1-丙烷磷酸二乙酯;丙基膦酸二乙酯
英文名称
diethyl propylphosphonate
英文别名
diethyl n-propylphosphonate;Propylphosphonsaeure-diethylester;Diethyl-propylphosphonat;diethyl 1-propylphosphonate;Propanphosphonsaeurediaethylester;1-diethoxyphosphorylpropane
CAS
18812-51-6
化学式
C7H17O3P
mdl
MFCD00015139
分子量
180.184
InChiKey
RUIKOPXSCCGLOM-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:94-96°C 12mm
-
密度:1.0079 g/cm3 (20 ºC)
-
闪点:94-96°C/12mm
-
稳定性/保质期:
在常温常压下稳定,应避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):1.1
-
重原子数:11
-
可旋转键数:6
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
海关编码:2931900090
-
储存条件:常温下应密闭避光,并存放在通风干燥处。
SDS
| Name: | Diethyl 1-Propanephosphonate Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 18812-51-6 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 18812-51-6 | Diethyl 1-Propanephosphonate | ca. 100 | unlisted |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. The toxicological properties of this substance have not been fully investigated.
Inhalation:
Causes respiratory tract irritation. The toxicological properties of this substance have not been fully investigated. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use only in a chemical fume hood. Wash clothing before reuse.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 18812-51-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear colorless
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H17O3P
Molecular Weight: 180.091
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents, reducing agents, acids, bases.
Hazardous Decomposition Products:
Carbon monoxide, oxides of phosphorus, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 18812-51-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Diethyl 1-Propanephosphonate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 18812-51-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 18812-51-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 18812-51-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— diethyl (3-chloropropyl)phosphonate 23269-98-9 C7H16ClO3P 214.629 —— diethyl 3-iodo-n-propylphosphonate 112221-15-5 C7H16IO3P 306.081 二乙基(3-溴丙基)膦酸酯 Diethyl 3-bromopropylphosphonate 1186-10-3 C7H16BrO3P 259.08 乙氧基(丙基)次膦酸 ethoxypropylphosphonic acid 21921-96-0 C5H13O3P 152.13 甲基膦酸二乙酯 Diethyl methylphosphonate 683-08-9 C5H13O3P 152.13 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2-Oxo-2-propyl-1,3-dioxa-2-phosphiran 75310-24-6 C5H11O3P 150.114 —— Propylphosphonsaeure-tetramethylenester 92221-58-4 C7H15O3P 178.168 —— Propyl-2-oxo-1,3,6-trioxa-2-phospha-cyclooctan 92221-61-9 C7H15O4P 194.167 —— diethyl (1-iodopropyl)phosphonate 1293932-63-4 C7H16IO3P 306.081 —— (1-formylpropyl)phosphonic acid diethyl ester 32329-34-3 C8H17O4P 208.194 —— Diethyl [1-(methylsulfanyl)propyl]phosphonate 82989-33-1 C8H19O3PS 226.277
反应信息
-
作为反应物:参考文献:名称:一种正丙基磷酸环酐的绿色合成方法摘要:一种正丙基磷酸环酐的绿色合成方法。本发明属于聚合物合成领域。本发明的目的是为了解决现有1‑丙基磷酸环酐反应温度高、条件苛刻、副产品处理难,以及部分原材料难以获取导致限制生产和反应时间长、成本高的技术问题。方法:步骤1:向溶剂中加入乙醇钠、亚磷酸二乙酯的甲苯溶液和溴丙烷,反应完成后加水静置分层,油层经脱溶和减压蒸馏,得到1‑丙基磷酸二乙酯;步骤2:向1‑丙基磷酸二乙酯中加入氢溴酸进行水解反应,得到丙基磷酸;步骤3:向丙基磷酸中加入乙酸酐,脱水反应,得到正丙基磷酸酐。本发明的方法经简单处理即可完成副产物溴化钠的高效去除,且原料简单易得、且价格低廉,合成步骤简单,生产成本低,适合工业化生产。公开号:CN114716480A
-
作为产物:参考文献:名称:用于利用1-的CC键形成一种新的,有效的方法,2-和3-膦酰基取代从iodoalkylphosphonates衍生的基团和Ñ -Bu 3 SNH / ET 3乙/ O 2系统摘要:描述了利用衍生自碘代烷基膦酸酯的1-,2-和3-膦酰基取代的基团和催化或化学计量的n- Bu 3 SnH / Et 3 B / O 2试剂体系的新型,实用的高取代膦酸酯的合成方法。DOI:10.1016/s0040-4020(97)00405-5
-
作为试剂:描述:(+/-)-5-hydroxymethyl-3,5-dimethyl-4-(methoxymethoxy)-5H-thiophen-2-one 在 正丁基锂 、 1-丙基膦酸二乙酯 、 戴斯-马丁氧化剂 作用下, 以 六甲基磷酰三胺 、 二氯甲烷 为溶剂, 反应 1.0h, 生成 3,5-dimethyl-4-(methoxymethoxy)-5H-thiophen-2-one参考文献:名称:Novel route to 5-position vinyl derivatives of thiolactomycin: olefination versus deformylation摘要:Vinyl and diene derivatives of thiolactomycin have been prepared via Horner-Wadsworth-Emmons olefination from protected 5-formyl-3,5-dimethylthiotetronic acid. Several 4-position protecting groups and a variety of phosphonates were evaluated, with MOM protection and beta-ketophosphonates yielding the highest ratio of the desired product to deformylated product. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2006.03.058
文献信息
-
Synthesen von 2,3-Dioxoalkylphosphonaten und anderer neuartiger ?-Ketophosphonate sowie eines Phosphinopyruvamids ( = (Alkyloxyphosphinyl)pyruvamids)
-
Phosphonated Fluoroquinolones, Antibacterial Analogs Thereof, and Methods for the Prevention and Treatment of Bone and Joint Infections申请人:Delorme Daniel公开号:US20080287396A1公开(公告)日:2008-11-20The present invention relates to phosphonated fluoroquinolones, antibacterial analogs thereof, and methods of using such compounds. These compounds are useful as antibiotics for prevention and/or the treatment of bone and joint infections, especially for the prevention and/or treatment of osteomyelitis.
-
Nickel/Photoredox-Catalyzed Methylation of (Hetero)aryl Chlorides Using Trimethyl Orthoformate as a Methyl Radical Source作者:Stavros K. Kariofillis、Benjamin J. Shields、Makeda A. Tekle-Smith、Michael J. Zacuto、Abigail G. DoyleDOI:10.1021/jacs.0c02805日期:2020.4.22represents a valuable transformation, but typically requires harsh reaction conditions or reagents. We report a radical approach for the methylation of (hetero)aryl chlorides using nickel/photoredox catalysis wherein trimethyl orthoformate, a common laboratory solvent, serves as a methyl source. This method permits methylation of (hetero)aryl chlorides and acyl chlorides at an early and late stage with
-
Fragmentation-Related Phosphonylation of Nucleophiles Utilizing P-Alkyl 2,3-oxaphosphabicyclo[2.2.2]octene 3-oxide Precursors作者:Tamara Kovács、Laura Szandra Fülöp、György KeglevichDOI:10.1002/hc.21304日期:2016.3New P-alkyl 2,3-oxaphosphabicyclo-[2.2.2]octene 3-oxides were synthesized by the Bayer–Villiger oxidation of the corresponding 7-phosphanorbornene 7-oxides and were used as precursors for reactive alkylmetaphosphonates useful in the phosphonylation of alcohols. This is the first case that the reactivity of the two regioisomers formed by O-insertion was differentiated and that the fragmentation-related
-
Synthesis of Cyclopropane-Containing Phosphorus Compounds by Radical Coupling of Butenylindium with Iodo Phosphorus Compounds作者:Kensuke Kiyokawa、Itaru Suzuki、Makoto Yasuda、Akio BabaDOI:10.1002/ejoc.201001471日期:2011.4by-product to be inert to the reaction system. In addition, the transmetalation of a (cyclopropylmethyl)stannane and InBr3 provided the dibutenylindium bromide as a single product, which smoothly coupled with the unstable phosphonyl radical species from the iodo phosphorus compound. A photochemical method (UV irradiation) was also applied and accelerated the coupling reaction. The cyclopropylmethylated通过(环丙基甲基)锡烷和 InBr3 之间的金属转移反应制备的 α-或 β-碘代磷化合物(如碘膦酸酯、碘代膦氧化物和碘代硫代膦酸酯)与丁烯基的自由基偶联,得到相应的环丙基甲基化产物。自由基反应是由丁烯基铟产生的自由基物种在少量氧气的辅助下引发的。Butenylindium 不仅可用作环丙基甲基化试剂,还可用作自由基引发剂。为了成功偶联,使用了锡/铟金属转移,其中卤化锡副产物对反应系统呈惰性非常重要。此外,(环丙基甲基)锡烷和 InBr3 的金属转移提供了二丁烯基溴化铟作为单一产品,其与来自碘磷化合物的不稳定膦酰基物种顺利偶联。还应用了光化学方法(紫外线照射)并加速了偶联反应。产生的环丙基甲基化膦酸酯是 Horner-Wadsworth-Emmons 反应的良好中间体,并产生带有环丙烷部分的官能化烯烃。
表征谱图
-
氢谱1HNMR
-
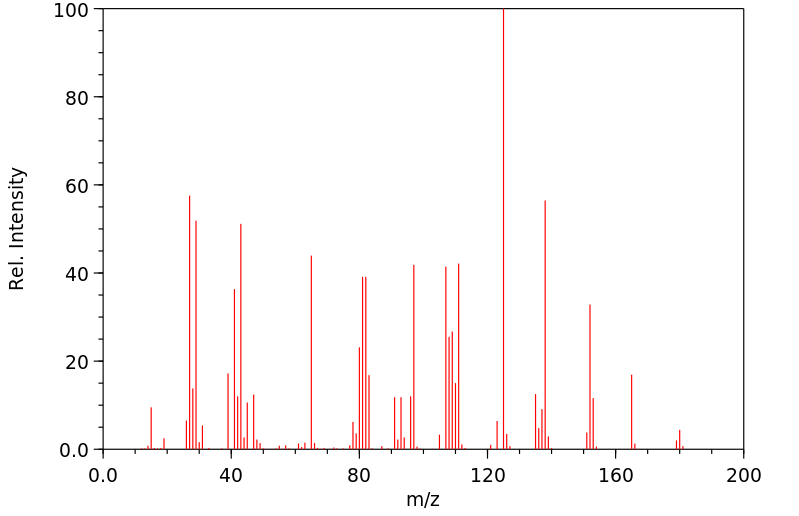
质谱MS
-
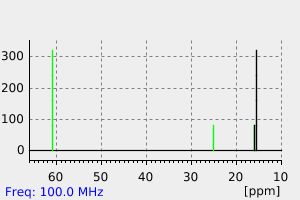
碳谱13CNMR
-
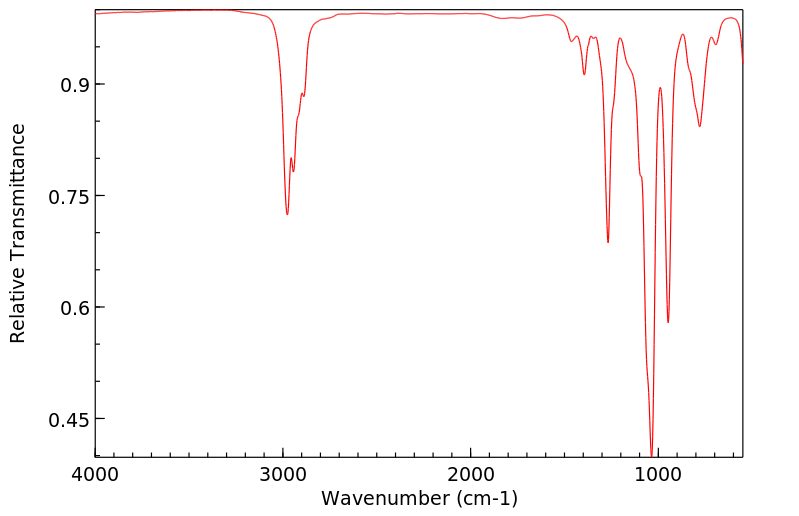
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(1-氨基丁基)磷酸
顺丙烯基磷酸
除草剂BUMINAFOS
阿仑膦酸
阻燃剂 FRC-1
铵甲基膦酸盐
钠甲基乙酰基膦酸酯
钆1,5,9-三氮杂环十二烷-N,N',N''-三(亚甲基膦酸)
钆-1,4,7-三氮杂环壬烷-N,N',N''-三(亚甲基膦酸)
重氮甲基膦酸二乙酯
辛基膦酸二丁酯
辛基膦酸
辛基-膦酸二钾盐
辛-1-烯-2-基膦酸
试剂12-Azidododecylphosphonicacid
英卡膦酸
苯胺,4-乙烯基-2-(1-甲基乙基)-
苯甲基膦酸二甲酯
苯基膦酸二甲酯
苯基膦酸二仲丁酯
苯基膦酸二乙酯
苯基膦酸二乙酯
苯基磷酸二辛酯
苯基二异辛基亚磷酸酯
苯基(1H-1,2,4-三唑-1-基)甲基膦酸二乙酯
Tetrapotassium (((2-hydroxyethyl)imino)bis(methylene))bisphosphonate
苄基膦酸苄基乙酯
苄基亚甲基二膦酸
膦酸,[(2-乙基己基)亚氨基二(亚甲基)]二,triammonium盐(9CI)
膦酸叔丁酯乙酯
膦酸单十八烷基酯钾盐
膦酸二辛酯
膦酸二(二十一烷基)酯
膦酸,辛基-,单乙基酯
膦酸,甲基-,单(2-乙基己基)酯
膦酸,甲基-,二(苯基甲基)酯
膦酸,甲基-,2-甲氧基乙基1-甲基乙基酯
膦酸,丁基乙基酯
膦酸,[苯基[(苯基甲基)氨基]甲基]-,二甲基酯
膦酸,[[羟基(苯基甲基)氨基]苯基甲基]-,二(苯基甲基)酯
膦酸,[2-(环丙基氨基)-2-羰基乙基]-,二乙基酯
膦酸,[2-(二甲基亚肼基)丙基]-,二乙基酯,(E)-
膦酸,[1-甲基-2-(苯亚氨基)乙烯基]-,二乙基酯
膦酸,[1-(乙酰基氨基)-1-甲基乙基]-(9CI)
膦酸,[(环己基氨基)苯基甲基]-,二乙基酯
膦酸,[(二乙氧基硫膦基)(二甲氨基)甲基]-
膦酸,[(2S)-2-氨基-2-苯基乙基]-,二乙基酯
膦酸,[(1Z)-2-氨基-2-(2-噻嗯基)乙烯基]-,二乙基酯
膦酸,P-[(二乙胺基)羰基]-,二乙基酯
膦酸,(氨基二环丙基甲基)-