1,1,2,2-四(4-甲基苯基)-1,2-乙二醇 | 913-86-0
中文名称
1,1,2,2-四(4-甲基苯基)-1,2-乙二醇
中文别名
1,1,2,2-四(4-甲基苯)-1,2-乙二醇
英文名称
tetra(4-methylphenyl)-1,2-ethanediol
英文别名
1,1,2,2-tetra-p-tolylethane-1,2-diol;1,2-Ethanediol, 1,1,2,2-tetrakis(4-methylphenyl)-;1,1,2,2-tetrakis(4-methylphenyl)ethane-1,2-diol
CAS
913-86-0
化学式
C30H30O2
mdl
——
分子量
422.567
InChiKey
UWVQXJFNNKWNLZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:177 °C
计算性质
-
辛醇/水分配系数(LogP):6.3
-
重原子数:32
-
可旋转键数:5
-
环数:4.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:40.5
-
氢给体数:2
-
氢受体数:2
安全信息
-
储存条件:室温
SDS
1,1,2,2-Tetrakis(4-methylphenyl)-1,2-ethanediol
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,1,2,2-Tetrakis(4-methylphenyl)-1,2-ethanediol
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,1,2,2-Tetrakis(4-methylphenyl)-1,2-ethanediol
Percent: ....
CAS Number: 913-86-0
Synonyms: 1,1,2,2-Tetra-p-tolyl-1,2-ethanediol
Chemical Formula: C30H30O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:177°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 1,1,2,2-Tetrakis(4-methylphenyl)-1,2-ethanediol
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 1,1,2,2-Tetrakis(4-methylphenyl)-1,2-ethanediol
Percent: ....
CAS Number: 913-86-0
Synonyms: 1,1,2,2-Tetra-p-tolyl-1,2-ethanediol
Chemical Formula: C30H30O2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: Crystal- Powder
Colour: White - Almost white
Odour: No data available
pH: No data available
Melting point/freezing point:177°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
No data available
Lower:
Upper: No data available
No data available
Relative density:
Solubility(ies):
[Water] No data available
[Other solvents] No data available
Section 10. STABILITY AND REACTIVITY
Stable under proper conditions.
Chemical stability:
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Section 10. STABILITY AND REACTIVITY
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
Not listed
UN-No:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:1,1,2,2-四(4-甲基苯基)-1,2-乙二醇 在 dichlorobis(dimethylsulfoxide)dioxomolybdenum(VI) 、 二甲基亚砜 作用下, 反应 0.17h, 以86%的产率得到4,4'-二甲基二苯甲酮参考文献:名称:一种选择性,高效且环保的乙二醇氧化裂解方法摘要:就环境影响而言,用于邻位二醇的氧化裂解的催化方法被描述为对涉及有毒氧化剂的传统方法的环境影响的有利替代方案。新颖的策略...DOI:10.1039/c5gc02862k
-
作为产物:描述:4,4'-二甲基二苯甲酮 在 甲醇 、 gallium nitride 作用下, 反应 12.0h, 以98%的产率得到1,1,2,2-四(4-甲基苯基)-1,2-乙二醇参考文献:名称:GaN纳米线作为可重复使用的光氧化还原催化剂,用于黑光照射下羰基的自由基偶联摘要:长期以来,利用光能驱动所需的化学转化。自由基偶联反应中均相光氧化还原催化剂的开发确实是惊人的,但是具有明显的缺点,例如难以分离催化剂和经常需要稀有贵金属。因此,我们设想使用具有可调费米能级和能带的超稳定III-V光敏半导体作为自由基耦合反应的易于隔离和可回收的异质光氧化还原催化剂。在本文中,使用羰基偶联反应作为概念验证,我们报道了在室温下,环境光下,以甲醇为溶剂和牺牲试剂,GaN纳米线催化的光频哪醇偶联反应。通过简单地调整掺杂剂,GaN纳米线显示出显着增强的电子性能。该催化剂表现出优异的稳定性,可重复使用性和功能耐受性。所有反应都可以在Si晶圆上用单条纳米线完成。DOI:10.1039/d0sc02718a
文献信息
-
CBZ6 as a Recyclable Organic Photoreductant for Pinacol Coupling作者:Hua Wang、Jian-Ping Qu、Yan-Biao KangDOI:10.1021/acs.orglett.1c00537日期:2021.4.16% CBZ6)-catalyzed reductive (pinacol) coupling of aldehydes, ketones, and imines has been developed. Irradiated by purple light (407 nm) using triethylamine as an electron donor, a variety of 1,2-diols and 1,2-diamines could be prepared. The oxidation potential of the excited state of CBZ6 is established as −1.92 V (vs saturated calomel electrode (SCE)). The relative high reductive potential enables
-
Visible Light Induced Reduction and Pinacol Coupling of Aldehydes and Ketones Catalyzed by Core/Shell Quantum Dots作者:Zi-Wei Xi、Lei Yang、Dan-Yan Wang、Chuan-Wei Feng、Yufeng Qin、Yong-Miao Shen、Chaodan Pu、Xiaogang PengDOI:10.1021/acs.joc.0c02627日期:2021.2.5105 for the reduction to alcohol and pinacol formation, respectively, and are very stable so that they can be recycled for at least 10 times in the reactions without significant loss of catalytic activity. The additional advantages of this method include good functional group tolerance, mild reaction conditions, the allowance of selectively reducing aldehydes in the presence of ketones, and easiness我们提出了一种有效且通用的可见光驱动方法,以CdSe / CdS核/壳量子点作为光催化剂将芳基醛和酮化学选择性地转化为醇或频哪醇产品。在这些反应中,硫酚被用作质子和氢原子的供体,以及被激发的量子点(QD)的空穴陷阱。只需更改反应系统中的硫酚含量,即可将两种产物从一种转换为另一种。核/壳QD催化剂高效,周转数(TON)大于4×10 4和4×10 5分别还原成醇和频哪醇形成,并且非常稳定,因此它们可以在反应中循环至少10次,而不会明显降低催化活性。该方法的其他优点包括良好的官能团耐受性,温和的反应条件,允许在存在酮的情况下选择性还原醛以及易于大规模反应。通过猝灭实验和自由基捕获实验研究了反应机理,并给出了随着反应条件的变化反应路径发生切换的原因。
-
Light-enabled metal-free pinacol coupling by hydrazine作者:Zihang Qiu、Hanh D. M. Pham、Jianbin Li、Chen-Chen Li、Durbis J. Castillo-Pazos、Rustam Z. Khaliullin、Chao-Jun LiDOI:10.1039/c9sc03737c日期:——Efficient carbon–carbon bond formation is of great importance in modern organic synthetic chemistry. The pinacol coupling discovered over a century ago is still one of the most efficient coupling reactions to build the C–C bond in one step. However, traditional pinacol coupling often requires over-stoichiometric amounts of active metals as reductants, causing long-lasting metal waste issues and sustainability高效的碳-碳键形成在现代有机合成化学中非常重要。一个多世纪前发现的频哪醇偶联仍然是一步构建 C-C 键的最有效偶联反应之一。然而,传统频哪醇偶联通常需要过量化学计量的活性金属作为还原剂,从而导致长期存在的金属废料问题和可持续性问题。一个巨大的科学挑战是设计一种无金属的频哪醇偶联反应方法。在这里,我们描述了一种光驱动频哪醇耦合协议,不使用任何金属,但使用 N 2 H 4,用作清洁的非金属氢原子转移 (HAT) 还原剂。在这种转化中,只有无痕无毒的N 2和H 2气体作为副产物产生,具有相对广泛的芳香酮范围和良好的官能团耐受性。对该机制的实验和计算研究表明,这种新颖的频哪醇偶联反应是通过光激发酮和 N 2 H 4之间的 HAT 过程进行的,而不是金属还原剂的常见单电子转移 (SET) 过程。
-
Reductive cleavage of carbon-halogen bonds by tri-n-butyltin hydride, lithium 4,4'-dimethylbenzophenone ketyl, and metallic magnesium作者:James J. Barber、George M. WhitesidesDOI:10.1021/ja00521a037日期:1980.1Ratc-structurc prof ilcs arc comparcd for four rcactions: reductittn of alkyl chlorides and brornidcs with tri-n-butyltin hydridc (Et:O. -15 oC and 0 oC-. rcspcciivcll). rcducrion oi alky'l brornides with lithrum'1,4'-din rcthylbenzopheno nc tiHf.,24.a), and rcacrisn ol'alkllchloridc iwith mctallic nragncsiunr (t:t:O,0'C').All iour ratc structure profiles are lin- carly'currclatcd (corrclation coclRatc-structurc prof ilcs 比较了四种反应:烷基氯化物和溴化物与三正丁基锡氢化物的还原(Et:O. -15 oC 和 0 oC-. rcspcciivcll)。rcducrion oi alky'l bronides with lithrum'1,4'-din rcthylbenzopheno nc tiHf.,24.a), 和 rcacrisn ol'alkllchloridc iwith mctallic nragncsiunr (t:t:O,0'C'). 所有的大鼠结构配置文件是线性的'currclatcd (corrclation cocl.l'icicnt r ) 0.ttO)。这些曲线的相似性表明碳卤键在转换状态或反应的每个类型中是显着的。(:lcctrochcrnica l rcduction potcntials of an linlitcd nunlbcr
-
Novel Reduction of Carbonyl Compounds with Al/NH<sub>3</sub>/Halide under Irradiation of Ultrasonic Wave作者:Ryu Sato、Takeshi Nagaoka、Takehiko Goto、Minoru SaitoDOI:10.1246/bcsj.63.290日期:1990.1Various carbonyl compounds, such as benzophenones and acetophenones, were reduced by Al/NH3/halide under ultrasonic wave irradiation to give the corresponding monohydric alcohols and/or pinacols in satisfactory yields. The addition of inorganic halides improved the selectivity in the formation of monohydric alcohols and pinacols.
表征谱图
-
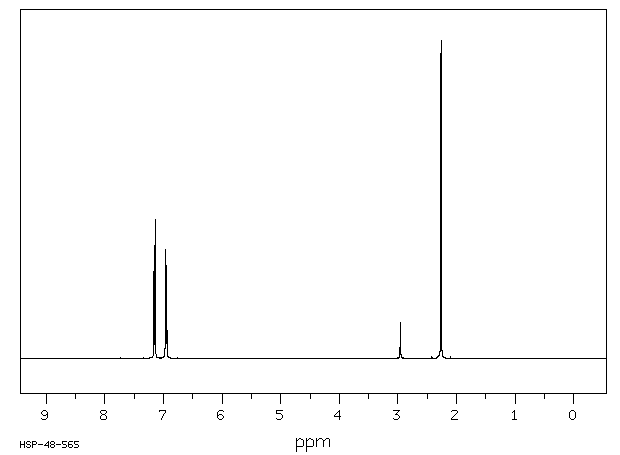
氢谱1HNMR
-
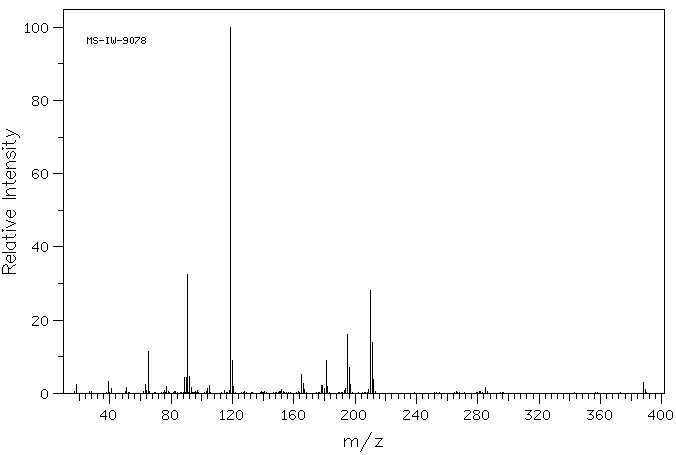
质谱MS
-
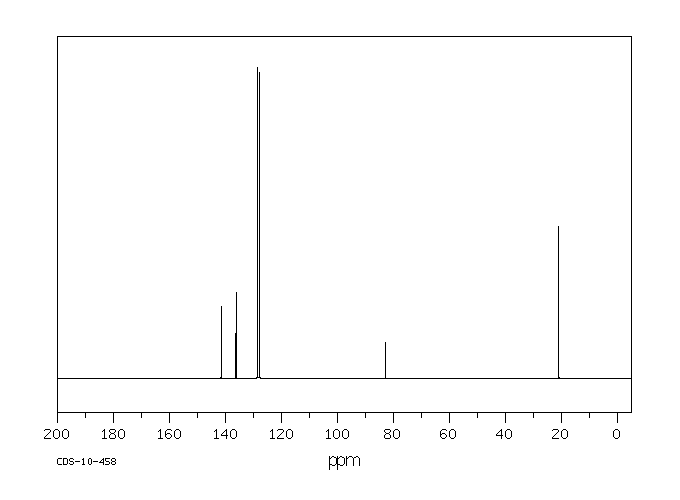
碳谱13CNMR
-
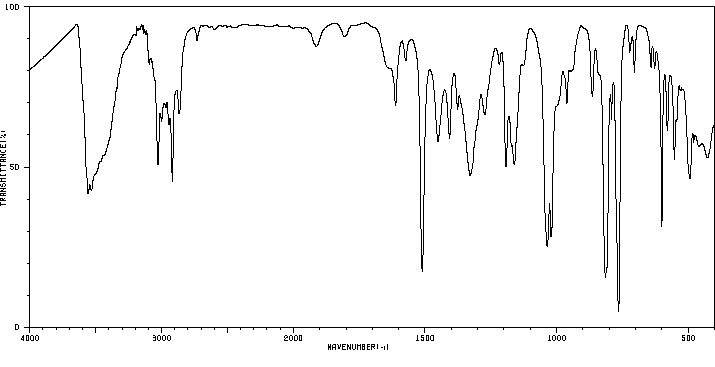
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸