4-氯-2,6-二甲基苯酚 | 1123-63-3
物质功能分类
中文名称
4-氯-2,6-二甲基苯酚
中文别名
4-氯-2,6-二甲酚
英文名称
2,6-Dimethyl-4-chlorophenol
英文别名
4-chloro-2,6-dimethylphenol
CAS
1123-63-3
化学式
C8H9ClO
mdl
MFCD00020138
分子量
156.612
InChiKey
VWYKSJIPZHRLNO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:80-85 °C
-
沸点:217.76°C (rough estimate)
-
密度:1.0950 (rough estimate)
-
溶解度:0.02 M
-
保留指数:1313.1
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会分解,也未有已知的危险反应。应避免与氧化物、碱、酸酐及酸性氯化物接触。
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险品运输编号:25kgs
-
海关编码:2908199090
-
安全说明:S26,S37/39
-
危险性防范说明:P261,P280,P301+P312,P302+P352,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将贮藏器保持密封,并存放在阴凉、干燥处。同时,确保工作环境有良好的通风或排气设施。
SDS
| Name: | 4-Chloro-2 6-Dimethylphenol Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 1123-63-3 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1123-63-3 | 4-Chloro-2,6-Dimethylphenol | ca. 100 | 214-376-0 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Wash mouth out with water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or chemical foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Provide ventilation. Vacuum or sweep up material and place into a suitable, dry disposal container.
Section 7 - HANDLING and STORAGE
Handling:
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 1123-63-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: Not available.
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 81 - 83 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H9ClO
Molecular Weight: 156.61
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Oxidizing agents, acid chlorides, acid anhydrides, bases, steel, copper.
Hazardous Decomposition Products:
Hydrogen chloride, carbon monoxide, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1123-63-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-Chloro-2,6-Dimethylphenol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 1123-63-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1123-63-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1123-63-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-氯-2-甲基苯酚 2-methyl-4-chlorophenol 1570-64-5 C7H7ClO 142.585 5-氯间二甲苯-2,alpha,alpha'-三醇 4-chloro-2,6-bis(hydroxymethyl)phenol 17026-49-2 C8H9ClO3 188.611 2,6-二甲基苯酚 2,6-xylenol 576-26-1 C8H10O 122.167 4-氯-2-[(5-氯-2-羟基-3-甲基苯基)甲基]-6-甲基苯酚 4,4'-dichloro-6,6'-dimethyl-2,2'-methanediyl-di-phenol 57693-35-3 C15H14Cl2O2 297.181 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-chloro-2-methoxy-1,3-dimethylbenzene 14804-27-4 C9H11ClO 170.639 2,6-二甲酰基-4-氯苯酚 2,6-diformyl-4-chlorophenol 32596-43-3 C8H5ClO3 184.579 2,6-二甲基苯酚 2,6-xylenol 576-26-1 C8H10O 122.167 —— 4-chloro-2,6-dimethylphenyl isopropyl ether 95729-57-0 C11H15ClO 198.692 —— 2-(4-Chlor-2,6-dimethyl-phenyoxy)-ethylamin —— C10H14ClNO 199.68 —— (4-Chloro-2,6-dimethyl-phenoxy)-acetonitrile 303224-73-9 C10H10ClNO 195.648 —— 1-(4-chloro-2,6-dimethyl-phenoxy)-propan-2-ol 69557-49-9 C11H15ClO2 214.692 —— 1-(4-chloro-2,6-dimethylphenoxy)propan-2-one 442628-90-2 C11H13ClO2 212.676 —— 3-(4-chloro-2,6-dimethyl-phenoxy)-propane-1,2-diol 69557-47-7 C11H15ClO3 230.691 1-[2-(4-氯-2,6-二甲基苯氧基)乙基]胍 N-[2-(4-Chlor-2,6-dimethyl-phenoxy)-ethyl]-guanidin 67227-39-8 C11H16ClN3O 241.721 —— methyl 2-(4-chloro-2,6-dimethylphenoxy)acetate 787575-74-0 C11H13ClO3 228.675 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:芳基阳离子和卡宾中间体在氯酚的光脱卤中。摘要:通过产物研究和瞬态吸收光谱法研究了2,6-二甲基-4-氯苯酚(6)在甲醇和三氟乙醇(TFE)中的光化学反应。来自三重态6的氯化物损失产生三重态羟苯基阳离子14,其在几十纳秒内与三重态氧代环己二烯基15平衡。但是,阳离子可以被烯丙基三甲基硅烷(k(ad)= 10(8)-10(9)m(-1)s(-1))选择性捕获,从而得到a离子和烯丙基化苯酚。在纯醇中,14和15的还原机理不同,即分别通过自由基阳离子17和苯氧基16进行氢转移。通过对O-甲硅烷基化衍生物的平行研究证实了机械合理性。这项工作表明,高(但选择性)反应性苯基阳离子14的化学不仅可以与同样的高反应性卡宾15区别开来,而且还可以用于合成有用的反应,如在这种情况下与烯烃的反应。给电子取代的卤代苯的光解可能是温和生成某些类别的苯基阳离子的一种选择方法。DOI:10.1002/chem.200400681
-
作为产物:描述:4-氯-2-甲基苯酚 在 chlorobis(ethylene)rhodium(I) dimer 、 硫酸 、 氢气 、 三乙胺 作用下, 以 1,4-二氧六环 、 溶剂黄146 、 乙腈 为溶剂, 20.0~150.0 ℃ 、1.03 MPa 条件下, 反应 24.0h, 生成 4-氯-2,6-二甲基苯酚参考文献:名称:2,2'-亚甲基二酚中无应变 C(芳基)–C(烷基)键的催化活化摘要:无应变和非极性 C-C 键的催化活化仍然是一个很大程度上未解决的挑战。在这里,我们描述了我们在开发铑催化氢解 2,2'-亚甲基二酚中无应变 C(芳基)-C(烷基)键的详细努力,并辅以可去除的定向基团。通过耐受广泛的官能团,获得了良好的单酚产品收率。此外,该反应具有可扩展性,催化剂负载量可降至 0.5 mol%。此外,该方法被证明可有效切割酚醛树脂和商用酚醛清漆树脂模型中的 C(芳基)-C(烷基)键。最后,详细的实验和计算机制研究表明,C-H 活化是一种竞争性但可逆的非循环反应,DOI:10.1021/jacs.1c13342
-
作为试剂:描述:2-甲基丙烯醛 、 2,2,2-trifluoro-1-(4-methylphenyl)-N-[(4-nitrophenyl)methyl]ethanimine 在 4-氯-2,6-二甲基苯酚 、 C60H58ClN4O4(1+)*Br(1-) 、 potassium hydroxide 、 sodium tetrahydroborate 、 盐酸 作用下, 以 水 、 甲苯 为溶剂, 反应 1.0h, 以77%的产率得到参考文献:名称:Control of chemoselectivity in asymmetric tandem reactions: Direct synthesis of chiral amines bearing nonadjacent stereocenters摘要:本文描述了一种机理洞察引导下的催化剂系统的开发,采用酚质子供体催化剂以及金刚烷衍生相转移催化剂,来控制两种不同中间体的化学选择性,从而使所需的不对称串联共轭加成-质子化途径主导于许多副反应途径,提供了一种合成方法,直接生成带有两个非相邻立体中心的光学活性胺。DOI:10.1073/pnas.1718474115
文献信息
-
[EN] N-ACYLHYDRAZONE DERIVATIVES FOR SELECTIVE T CELL INHIBITOR AND ANTI-LYMPHOID MALIGNANCY DRUG<br/>[FR] DÉRIVÉS DE N-ACYLHYDRAZONE DESTINÉS À UN MÉDICAMENT ANTIMALIGNITÉ LYMPHOÏDE ET INHIBANT SÉLECTIVEMENT LES LYMPHOCYTES T申请人:CHONG KUN DANG PHARM CORP公开号:WO2014035149A1公开(公告)日:2014-03-06The present invention relates to novel N-acylhydrazone derivatives, and more particularly to novel N-acylhydrazone derivatives having selective T cell inhibitory activity and/or anti-lymphoid malignancy activity, stereoisomers thereof, pharmaceutically acceptable salts thereof, the use thereof for preparing pharmaceutical compositions, pharmaceutical compositions containing the same, treatment methods using the compositions, and methods for preparing the novel N-acylhydrazone derivatives.
-
A Dearomatization/Debromination Strategy for the [4+1] Spiroannulation of Bromophenols with α,β‐Unsaturated Imines作者:Yicong Ge、Cheng Qin、Lu Bai、Jiamao Hao、Jingjing Liu、Xinjun LuanDOI:10.1002/anie.202008130日期:2020.10.19A novel [4+1] spiroannulation of o‐ & p‐bromophenols with α,β‐unsaturated imines has been developed for the direct synthesis of a new family of azaspirocyclic molecules. Notably, several other halophenols (X=Cl, I) were also applicable for this transformation. Moreover, a catalytic asymmetric version of the reaction was realized with 1‐bromo‐2‐naphthols by using a chiral ScIII/Py‐Box catalyst. Mechanistic
-
Design, synthesis and anti-HIV evaluation of novel diarylpyridine derivatives as potent HIV-1 NNRTIs作者:Zhaoqiang Liu、Ye Tian、Jinghan Liu、Boshi Huang、Dongwei Kang、Erik De Clercq、Dirk Daelemans、Christophe Pannecouque、Peng Zhan、Xinyong LiuDOI:10.1016/j.ejmech.2017.07.012日期:2017.11“me-better” drugs of DAPYs, novel diarylpyridine derivatives were designed, synthesized and evaluated for their anti-HIV activities in MT-4 cells. The majority of these compounds showed high activity against wild-type HIV-1 strain (IIIB) with EC50 values in the range of 0.04–4.41 μM. Among them, compound 5b2 (EC50 = 0.04 μM, SI = 3963) was the most potent. This compound showed anti-HIV-1IIIB activity
-
Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 2: Discovery of novel [1,2,4]Triazolo[1,5-a]pyrimidines using a structure-guided core-refining approach作者:Liu Wang、Ye Tian、Wenmin Chen、Hong Liu、Peng Zhan、Dongyue Li、Huiqing Liu、Erik De Clercq、Christophe Pannecouque、Xinyong LiuDOI:10.1016/j.ejmech.2014.07.104日期:2014.10Guided by crystal structures of HIV-1 RT/DAPY complex and molecular modeling studies, a series of novel [1,2,4]triazolo[1,5-a]pyrimidine derivatives were rationally designed via structure-based core refining approach, synthesized through the readily accessible synthetic methods and evaluated for their anti-HIV activities in MT-4 cells. Preliminary biological evaluation indicated that most of the compounds在HIV-1 RT / DAPY复合物的晶体结构和分子建模研究的指导下,通过基于结构的核心精制方法合理设计了一系列新颖的[1,2,4]三唑并[1,5-a]嘧啶衍生物,合成了通过容易获得的合成方法,并评估了它们在MT-4细胞中的抗HIV活性。初步生物学评估表明,大多数化合物对野生型HIV-1 III B表现出明显的抑制活性。特别是,化合物7n是针对HIV-1的野生型和K103N / Y181C双抗突变株的最有效抑制剂,其EC 50值分别为0.02μM和7.6μM,远优于或类似于奈韦拉平(NVP) ,EC50 = 0.15μM,2.9μM)和地拉夫定(DLV,EC 50 = 0.07μM,> 36μM)。此外,其他一些化合物5b,7c,7e,7f和7m也具有良好的抗HIV-1效能(分别为EC 50 = 0.07、0.05、0.05、0.07和0.05μM),效果更好与NVP和DLV的相似
-
Fused heterocycles bearing bridgehead nitrogen as potent HIV-1 NNRTIs. Part 4: Design, synthesis and biological evaluation of novel imidazo[1,2-a]pyrazines作者:Boshi Huang、Xin Liang、Cuicui Li、Wenmin Chen、Tao Liu、Xiao Li、Yueyue Sun、Lu Fu、Huiqing Liu、Erik De Clercq、Christophe Pannecouque、Peng Zhan、Xinyong LiuDOI:10.1016/j.ejmech.2015.02.022日期:2015.3structure-guided core-refining approach, a series of novel imidazo[1,2-a]pyrazine derivatives were designed, synthesized and evaluated as HIV-1 non-nucleoside reverse transcriptase inhibitors (NNRTIs). Biological results of antiviral assay in MT-4 cell cultures showed that 12 target compounds displayed moderate activities against wild-type (wt) HIV-1 strain (IIIB) with EC50 values ranging from 0.26 μM to通过结构导向的核心提纯方法,设计,合成和评估了一系列新型的咪唑并[1,2- a ]吡嗪衍生物,并将其作为HIV-1非核苷逆转录酶抑制剂(NNRTIs)进行评估。MT-4细胞培养物中抗病毒测定的生物学结果表明,有12种目标化合物对野生型(wt)HIV-1菌株(III B)表现出中等活性,EC 50值为0.26μM至19μM。其中4a和5a是两个活性最高的类似物,其EC 50值分别为0.26μM和0.32μM,可与地拉夫定(DLV,EC 50 = 0.54μM)和奈韦拉平(NVP,EC 50)相提并论。 = 0.31μM)。另外,有9种化合物的RT抑制活性优于NVP。此外,详细讨论了一些代表性化合物4a和5a的预测类药物性质,以及结构-活性关系(SAR)分析。通过分子模拟研究了化合物4a的结合模式。
表征谱图
-
氢谱1HNMR
-
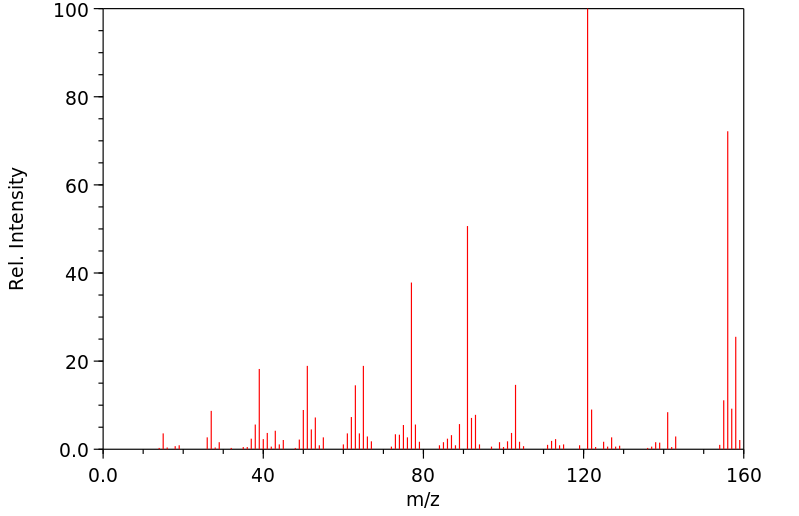
质谱MS
-
碳谱13CNMR
-
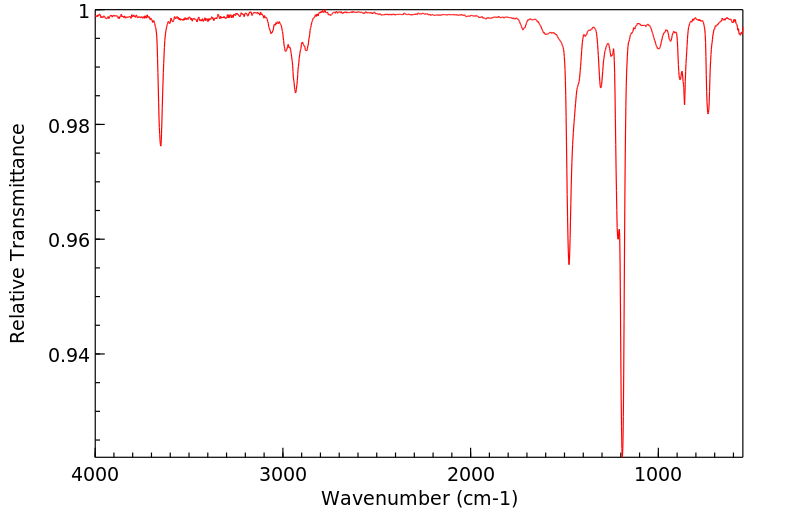
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-氯-6-羟基苯基)硼酸
黄柄曲菌素
高香草酸-d3
高香草酸-13C6
高香草酸
高香兰酸乙酯
高辣椒素II
高二氢辣椒素I
香草醛醛肟
香草醛苯腙
香草醛-甲氧基-13C
香草醛-(N-对甲苯基肟)
香草醛
香草酸肼
香草壬酰胺
香草基扁桃酸乙酯
香草吗啉
香草二乙胺
香兰素胺硬脂酸盐
香兰素胺硬脂酸盐
香兰素胺盐酸盐
香兰素丙二醇缩醛
香兰素13C6
香兰素-D3
香兰基乙基醚
香兰基丁醚
顺式-5-正十五碳-8'-烯基间苯二酚
顺式-1-(2-羟基-5-甲基苯基)-2-丁烯-1-酮
顺式-1-(2-羟基-4-甲氧基苯基)-2-丁烯-1-酮
顺-3-氯二氢-5-苯基呋喃-2(3H)-酮
雌二醇杂质1
降二氢辣椒碱
阿诺洛尔
阿瓦醇
阿普斯特杂质
间苯二酚双(二苯基磷酸酯)
间苯二酚-烯丙醇聚合物
间苯二酚-D6
间苯二酚
间苯三酚甲醛
间苯三酚二水合物
间苯三酚
间羟基苯乙基溴
间硝基苯酚
间甲酚紫钠盐
间甲酚与对甲酚和苯酚甲醛树脂的聚合物
间甲酚-D7
间甲酚-D3
间甲酚
间溴苯酚