(S)-(-)-1,2,3,4-四氢-3-异喹啉甲醇 | 18881-17-9
中文名称
(S)-(-)-1,2,3,4-四氢-3-异喹啉甲醇
中文别名
(S)-1,2,3,4-四氢异喹啉-3-基-甲醇;(S)-1,2,3,4-四氢异喹啉-3-甲醇;(S)-1,2,3,4-四氢-3-异喹啉甲醇;(S)-3-羟甲基-1,2,3,4-四氢异喹啉;L-1,2,3,4-四氢-3-异喹啉甲醇
英文名称
(S)-3-hydroxymethyl-1,2,3,4-tetrahydroisoquinoline
英文别名
(3S)-1,2,3,4-tetrahydroisoquinolin-3-ylmethanol;(S)-(-)-1,2,3,4-tetrahydroisoquinoline-3-methanol;(S)-1,2,3,4-Tetrahydroisoquinoline-3-methanol;[(3S)-1,2,3,4-tetrahydroisoquinolin-3-yl]methanol
CAS
18881-17-9
化学式
C10H13NO
mdl
——
分子量
163.219
InChiKey
ZSKDXMLMMQFHGW-JTQLQIEISA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:114-116 °C(lit.)
-
沸点:290.31°C (rough estimate)
-
密度:1.0508 (rough estimate)
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:12
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.4
-
拓扑面积:32.3
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933499090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:存于阴凉干燥处。
SDS
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 1. 化学品
产品名称: (S)-1,2,3,4-Tetrahydroisoquinoline-3-methanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (S)-1,2,3,4-四氢异喹啉-3-甲醇
百分比: >95.0%(T)
CAS编码: 18881-17-9
俗名: (S)-3-Hydroxymethyl-1,2,3,4-tetrahydroisoquinoline
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 3. 成分/组成信息
分子式: C10H13NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
114°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: (S)-1,2,3,4-Tetrahydroisoquinoline-3-methanol
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
脱掉被污染的衣物,清洗后方可重新使用。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (S)-1,2,3,4-四氢异喹啉-3-甲醇
百分比: >95.0%(T)
CAS编码: 18881-17-9
俗名: (S)-3-Hydroxymethyl-1,2,3,4-tetrahydroisoquinoline
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 3. 成分/组成信息
分子式: C10H13NO
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 9. 理化特性
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点:
114°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
(S)-1,2,3,4-四氢异喹啉-3-甲醇 修改号码:5
模块 14. 运输信息
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,4-四氢异喹啉-3-羧酸 (R,S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid 67123-97-1 C10H11NO2 177.203 (S)-(-)-1,2,3,4-四氢异喹啉-3-羧酸 L-Tic-OH 74163-81-8 C10H11NO2 177.203 —— methyl (3S)-1,2,3,4-tetrahydro-3-isoquinolinecarboxylate 79815-19-3 C11H13NO2 191.23 1,2,3,4-四氢异喹啉-3-羧酸乙酯 L-3-carbethoxy-1,2,3,4-tetrahydro-2H-isoquinoline 15912-55-7 C12H15NO2 205.257 S-1,2,3,4-四氢异喹啉-3-羧酸苄酯盐酸盐 benzyl (S)-1,2,3,4-tetrahydroisoquinoline-3-carboxylate 77497-96-2 C17H17NO2 267.327 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-(R)-甲基-1,2,3,4-四氢异喹啉 (3R)-3-methyl-1,2,3,4-tetrahydroisoquinoline 179893-97-1 C10H13N 147.22 —— (S)-N,N-dimethyl-2-((1,2,3,4-tetrahydroisoquinolin-3-yl)methoxy)ethanamine 1415099-51-2 C14H22N2O 234.341 —— (S)-N-methyl-3-hydroxymethyl-1,2,3,4-tetrahydroisoquinoline 54625-68-2 C11H15NO 177.246 —— (S)-3-Trimethylsilanyloxymethyl-1,2,3,4-tetrahydro-isoquinoline 1025931-48-9 C13H21NOSi 235.401 —— O-carbamoyl-(L)-3-oxymethyl-1,2,3,4-tetrahydroisoquinoline 243858-56-2 C11H14N2O2 206.244 —— (S)-(+)-<(1,2,3,4-tetrahydro-2-formyl)isoquinolin-3-yl>methanol 144564-18-1 C11H13NO2 191.23 —— (3S)-3-(tert-butyldimethylsilyloxymethyl)-1,2,3,4-tetrahydroisoquinoline 215928-81-7 C16H27NOSi 277.482 —— (3S)-3-(hydroxymethyl)-N-(3-pentynyl)-1,2,3,4-tetrahydroisoquinoline 864421-15-8 C15H19NO 229.322 —— (1R,3S)-1-methoxymethyl-3-hydroxymethyl-1,2,3,4-tetrahydroisoquinoline 219640-74-1 C12H17NO2 207.272 —— (S)-1-(3-(hydroxymethyl)-3,4-dihydroisoquinolin-2(1H)-yl)ethanone 1415095-38-3 C12H15NO2 205.257 —— (3S)-3,4-dihydro-3-tert-butyldimethylsiloxymethyl-1(2H)-isoquinolinone 215928-83-9 C16H25NO2Si 291.466 —— (S)-1-(3-((2-(dimethylamino)ethoxy)methyl)-3,4-dihydroisoquinolin-2(1H)-yl)ethanone 1415095-40-7 C16H24N2O2 276.379 —— (3S)-N-(3-pentynyl)-1,2,3,4-tetrahydroisoquinoline-3-carbaldehyde 864421-17-0 C15H17NO 227.306 3(S)-3-羟甲基-3,4-二氢-1H-异喹啉-2-羧酸叔丁酯 tert-butyl (S)-3-(hydroxymethyl)-3,4-dihydroisoquinoline-2(1H)-carboxylate 183958-71-6 C15H21NO3 263.337 —— [(2'S,3S)-2-(2'-benzyloxy-5'-methylhex-4'-enyl)-1,2,3,4-tetrahydroisoquinolin-3-yl]methanol 782500-96-3 C24H31NO2 365.516 —— benzyl (3S)-3-(hydroxymethyl)-3,4-dihydro-1H-isoquinoline-2-carboxylate 195832-14-5 C18H19NO3 297.354 —— Boc-L-Tic-CH2OMe 357339-26-5 C16H23NO3 277.364 - 1
- 2
反应信息
-
作为反应物:描述:(S)-(-)-1,2,3,4-四氢-3-异喹啉甲醇 在 咪唑 、 碳酸氢钠 作用下, 以 四氢呋喃 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 生成 (3S)-2-carboethoxy-1,2,3,4-tetrahydro-3-tert-butyldimethylsiloxymethylisoquinoline参考文献:名称:Preparation and Diastereoselective Birch Reduction−Alkylation of Chiral 3,4-Dihydro-1(2H)-isoquinolinones. Enantiospecific Syntheses and Opioid Receptor Affinities of Several Hydro-2,3- dimethyl-1H-7,12a-methanobenzo[6,7]cycloocta[1,2-c]pyridine-9-ols摘要:Synthetic procedures have been developed to provide 2,3-disubstituted-3,4-dihydro-1(2H)-isoquinones 6, 10, and 15 from (1R,2S)-ephedrine, (1R,2R)-pseudoephedrine, and L-phenylalanine. Birch reduction of 6 and 10 gave enantiomerically related lactam enolates that were alkylated with methyl iodide, allyl bromide, benzyl bromide, p-benzyloxybenzyl bromide, and p-methoxybenzyl bromide to give 7a-7e, 11a, and 11b with diastereoselectivities > 20:1. Birch reduction-methylation of 15 gave 19 with a diastereoselectivity of >35:1. Selective reduction of the disubstituted double bond in 19 with diimide and cleavage of the tert-butyldimethylsilyl ether gave 20b, from which iodoetherification under thermodynamic control gave the iodopyran 21a; iodoetherification of 20b under kinetic control gave the iodotetrahydrofuran 22. Enantiospecific syntheses of analogues of 24 (Schultz, A. G.; Kirincich, S. J.; Rahm, R. Tetrahedron Lett. 1995, 36, 4551-4554) have been developed. Tetracycle 24 is isomeric with the potent analgesic agent levorphanol, but the bridging of the hydroisoquinoline ring by the hydroxybenzyl unit in 24 is at C(7, isoquinoline numbering) and C(8a) rather than at C(1) and C(4a) as in levorphanol. The key step in the transformation of 7d and 7e to tetracyclic phenolic amines (-)-26 and (+)-28 is the Grewe-type cyclization of 7d to 25b and 7e to 25c. K-i values for the inhibition of binding to the mu-, delta-, and kappa-opioid receptors by (-)-26, (+)-26, (+)-28, (-)-28, and (+)-32 are reported.DOI:10.1021/jo980921g
-
作为产物:描述:S-1,2,3,4-四氢异喹啉-3-羧酸苄酯盐酸盐 在 lithium aluminium tetrahydride 作用下, 以 四氢呋喃 为溶剂, 生成 (S)-(-)-1,2,3,4-四氢-3-异喹啉甲醇参考文献:名称:芳香酮的对映选择性催化硼烷还原:(S)-卟啉的两种手性β-氨基醇的合成与应用摘要:在原位从两个光学活性的β氨基醇的手性形成恶唑硼烷催化剂(小号) - 1和(小号) - 2从苄基(小号)-1,2,3,4-四氢异喹啉-3-羧酸乙酯(小号) - 3已成功地用于各种芳族酮的对映选择性催化均相硼烷还原。DOI:10.1016/s0957-4166(00)80196-2
-
作为试剂:描述:苯乙酮 在 [RhCl2(p-cymene)]2 、 (S)-(-)-1,2,3,4-四氢-3-异喹啉甲醇 、 potassium tert-butylate 、 异丙醇 作用下, 反应 24.0h, 生成 (S)-(-)-1-苯乙醇 、 (R)-(+)-1-苯基乙醇参考文献:名称:用于不对称转移氢化反应的 C1-取代四氢异喹啉衍生物的合成与筛选摘要:四氢异喹啉 (TIQ) 衍生物表现出良好的生物活性。然而,TIQ 化合物在不对称催化中的应用是有限的。本文介绍了一系列用于不对称转移氢化 (ATH) 反应的 TIQ 衍生物。合成了手性 TIQ 氨基醇配体并筛选了芳香酮的 ATH 反应。通过实验和计算研究了 C-1 位置上的顺式和反式苯基取代对配体骨架的影响。结果表明,在室温下用 Ru 复合物获得的 TIQ 支架上的反式取向产生更高的周转率,选择性为 94% ee。顺式异构体导致没有选择性的高周转率。反式异构体在较低温度下产生 99% ee。此外,DOI:10.1002/ejoc.200901159
文献信息
-
SUBSTITUTED SULFONAMIDE COMPOUNDS申请人:OBERBOERSCH Stefan公开号:US20080153843A1公开(公告)日:2008-06-26Substituted sulfonamide derivatives, a process for their preparation, pharmaceutical compositions containing these compounds, and to the use of substituted sulfonamide derivatives in the treatment or inhibition of pain and/or various disorders or disease states.
-
Design, Synthesis, and Structure–Activity Relationships of Novel Tetrahydroisoquinolino Benzodiazepine Dimer Antitumor Agents and Their Application in Antibody–Drug Conjugates作者:Naidu S. Chowdari、Yong Zhang、Ivar McDonald、Walter Johnson、David R. Langley、Prasanna Sivaprakasam、Robert Mate、Tram Huynh、Srikanth Kotapati、Madhura Deshpande、Chin Pan、Daniel Menezes、Yichong Wang、Chetana Rao、Ganapathy Sarma、Bethanne M. Warrack、Vangipuram S. Rangan、Sung Mei-Chen、Pina Cardarelli、Shrikant Deshpande、David Passmore、Richard Rampulla、Arvind Mathur、Robert Borzilleri、Arvind Rajpal、Gregory Vite、Sanjeev GangwarDOI:10.1021/acs.jmedchem.0c01385日期:2020.11.25series of tetrahydroisoquinoline-based benzodiazepine dimers were synthesized and tested for in vitro cytotoxicity against a panel of cancer cell lines. Structure–activity relationship investigation of various spacers guided by molecular modeling studies helped to identify compounds with picomolar activity. Payload 17 was conjugated to anti-mesothelin and anti-fucosylated monosialotetrahexosylganglioside合成了一系列基于四氢异喹啉的苯并二氮杂二聚体,并测试了其对一组癌细胞系的体外细胞毒性。通过分子建模研究指导的各种间隔基的结构-活性关系研究有助于鉴定具有皮摩尔活性的化合物。有效负载17通过溶酶体可裂解的缬氨酸-瓜氨酸二肽接头,通过异源赖氨酸偶联和细菌转谷氨酰胺酶介导的位点特异性偶联,将其与抗间皮素和抗岩藻糖基化的单唾液酸四己糖基神经节苷脂(FucGM1)抗体偶联。在体外,这些抗体药物偶联物(ADC)对人类癌细胞系表现出显着的细胞毒性和靶标介导的选择性。在小鼠的胃癌和肺癌异种移植模型中,进一步评估了这些ADC的药代动力学和功效。在单剂量ADC- 46(0.02μmol / kg)之后,在这些模型中观察到一致的药代动力学特征,高靶标特异性和强大的抗肿瘤活性。
-
[EN] SUBSTITUTED SULFONAMIDES USEFUL AS ANTIAPOPTOTIC BCL INHIBITORS<br/>[FR] SULFONAMIDES SUBSTITUÉS UTILES COMME INHIBITEURS DE BCL ANTI-APOPTOTIQUES申请人:BRISTOL MYERS SQUIBB CO公开号:WO2012162365A1公开(公告)日:2012-11-29Disclosed are compounds of Formula (I), or a pharmaceutically acceptable salt thereof, wherein: W and Q and G are defined herein. Also disclosed are methods of using such compounds as inhibitors of Bcl-2 family antiapoptotic proteins for the treatment of cancer; and pharmaceutical compositions comprising such compounds.揭示了式(I)的化合物或其药学上可接受的盐,其中:W、Q和G在此处被定义。还揭示了将这些化合物用作Bcl-2家族抗凋亡蛋白的抑制剂以治疗癌症的方法;以及包含这些化合物的药物组合物。
-
Substituted Sulfonamide Compounds申请人:OBERBOERSCH Stefan公开号:US20080249128A1公开(公告)日:2008-10-09Substituted sulfonamide compounds with bradykinin receptor (B1R) modulating activity; processes for the preparation thereof, pharmaceutical compositions comprising such compounds, and methods of using such compounds to treat or inhibit pain and/or other disorders and/or disease states.用具有激肽酶受体(B1R)调节活性的磺胺化合物替代;制备这些化合物的方法,包括这些化合物的药物组合物,以及使用这些化合物治疗或抑制疼痛和/或其他疾病状态的方法。
-
Efficient Access to New Chemical Space Through Flow-Construction of Druglike Macrocycles Through Copper-Surface-Catalyzed Azide-Alkyne Cycloaddition Reactions作者:Andrew R. Bogdan、Keith JamesDOI:10.1002/chem.201002215日期:2010.12.27of 12‐ to 22‐membered macrocycles, with druglike functionality and properties, have been generated by using a simple and efficient copper‐catalyzed azide–acetylene cycloaddition reaction, conducted in flow in high‐temperature copper tubing, under environmentally friendly conditions. The triazole‐containing macrocycles have been generated in up to 90 % yield in a 5 min reaction, without resorting to
表征谱图
-
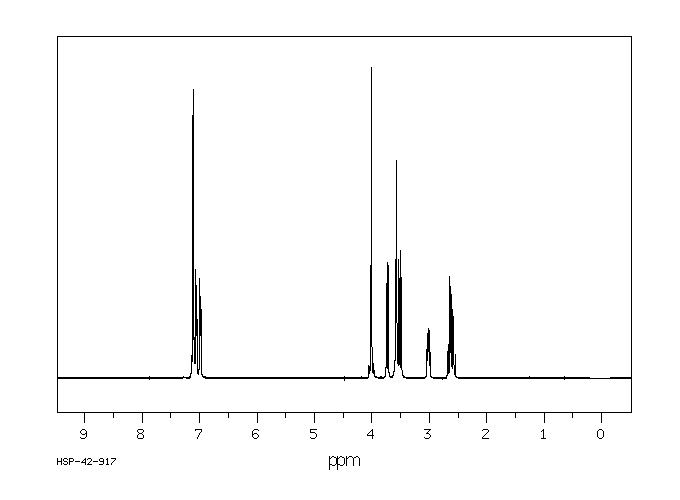
氢谱1HNMR
-
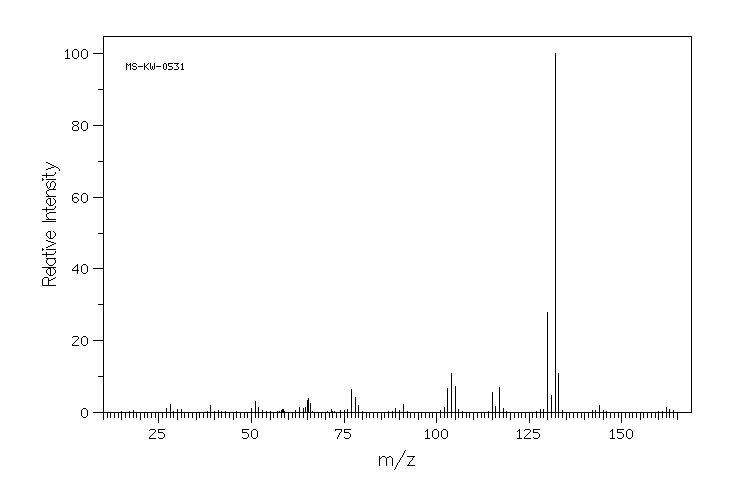
质谱MS
-
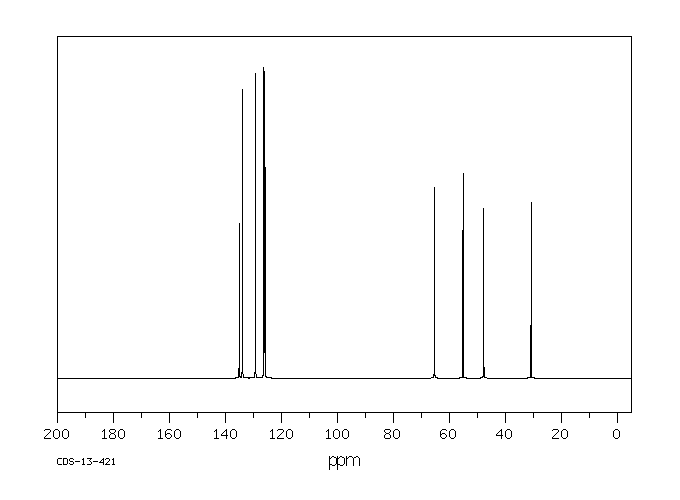
碳谱13CNMR
-
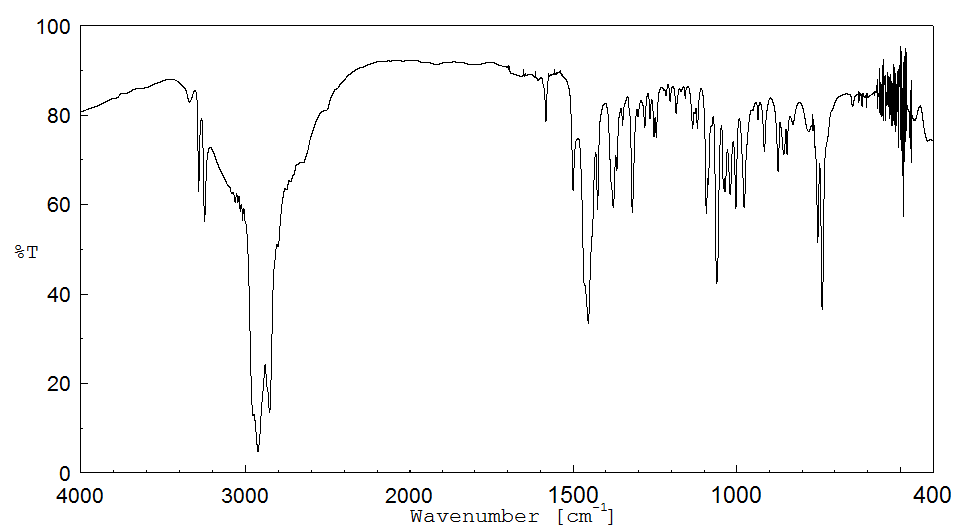
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-2-(环丁基氨基)-N-(3-(3,4-二氢异喹啉-2(1H)-基)-2-羟丙基)异烟酰胺
麦托福
鹿尾草定
酒石酸索利那新
车瑞灵
贝诺利嗪
诺米芬辛
螺苄基异喹啉
螺[异喹啉-4(4H),4-哌啶]-2(3H)-羧酸1,1-二甲基乙酯盐酸盐
蓝堇辛
萼卷豆碱
荷苞牡丹碱甲氧化物
苯喹胺
苯二甲酸可他寧
苄基7-溴-3,4-二氢-2(1H)-异喹啉羧酸酯
苄喹酰胺
胍尼索喹硫酸盐
羧基猪毛菜酚
紫堇杷灵碱
索非那新EP杂质H
索非那新
索喹洛尔
索利那新盐酸盐
索利那新杂质32
索利那新杂质29
索利那新杂质20
索利那新杂质2
索利那新杂质18
索利那新杂质17
索利那新杂质
索利那新-索非那新杂质
索利那新-d5盐酸盐
素立芬新
立他司特杂质19
硫酸异喹胍
直立角茴香碱
盐酸瑞伐拉赞
盐酸猪毛菜定
盐酸氯化苯喹胺
盐酸屈他维林
盐酸屈他维林
白毛莨分碱盐酸盐
甲基6-羟基-7-甲氧基-1,2,3,4-四氢异喹啉-1-羧酸酯
琥珀酸索利那辛
环戊羧酸,2-氨基-4-亚甲基-,(1R,2S)-(9CI)
环丙基(5,6,7,8-四氢咪唑并[1,2-a]吡嗪-2-基)甲酮
猪毛菜定
特布他林杂质B
潘红胺
溴胍喹定