2-碘己烷 | 18589-27-0
中文名称
2-碘己烷
中文别名
——
英文名称
2-iodohexane
英文别名
2-Jod-hexan;2-hexyl iodide;2-Iodhexan
CAS
18589-27-0
化学式
C6H13I
mdl
——
分子量
212.074
InChiKey
XCEDNORUDUKWGK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-68.15°C (estimate)
-
沸点:174.5°C (estimate)
-
密度:1.4121
-
保留指数:1037;973
计算性质
-
辛醇/水分配系数(LogP):3.9
-
重原子数:7
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903399090
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:BiX 3作为卤素交换反应的有效和选择性试剂摘要:卤化铋是在温和条件下进行的卤素交换反应中有效的选择性试剂。这种快速,高产率的反应主要在保持构型的情况下进行。DOI:10.1016/s0040-4020(98)01213-7
-
作为产物:描述:参考文献:名称:Pringsheim; Gorgas, Chemische Berichte, 1924, vol. 57, p. 1566摘要:DOI:
文献信息
-
Aliphatic C–H Bond Iodination by a <i>N</i>-Iodoamide and Isolation of an Elusive <i>N</i>-Amidyl Radical作者:Alexander Artaryan、Artur Mardyukov、Kseniya Kulbitski、Idan Avigdori、Gennady A. Nisnevich、Peter R. Schreiner、Mark GandelmanDOI:10.1021/acs.joc.7b00557日期:2017.7.21Contrary to C–H chlorination and bromination, the direct iodination of alkanes represents a great challenge. We reveal a new N-iodoamide that is capable of a direct and efficient C–H bond iodination of various cyclic and acyclic alkanes providing iodoalkanes in good yields. This is the first use of N-iodoamide for C–H bond iodination. The method also works well for benzylic C–H bonds, thereby constituting
-
Direct Bromination and Iodination of Non-Activated Alkanes by Hypohalite Reagents作者:Thomas Wirth、Raúl MontoroDOI:10.1055/s-2005-865322日期:——The direct functionalisation of alkanes through bromination and iodination has been successfully achieved. The combination of stoichiometric mixtures of elemental halogen and sodium alkoxides leads to the formation of alkyl hypobromites and hypoiodites as reagents. The halogenation occurs without external photostimulation under thermal reaction conditions.
-
An Efficient and Facile Hydroiodination of Alkenes and Alkynes Using Polymethylhydrosiloxane–Iodine System作者:Biswanath Das、Yallamalla Srinivas、Harish Holla、Ravirala NarenderDOI:10.1246/cl.2007.800日期:2007.6.5A mild and efficient method has been developed for the synthesis of alkyl and alkenyl iodides from alkenes and alkynes using polymethylhydrosiloxane (PMHS) and iodine in chloroform at room temperat...
-
A New Coupling Reaction of Alkyl Iodides with Electron Deficient Alkenes Using Nickel Boride (cat.)−Borohydride Exchange Resin in Methanol作者:Tae Bo Sim、Jaesung Choi、Meyoung Ju Joung、Nung Min YoonDOI:10.1021/jo961751f日期:1997.4.1addition reaction of alkyl iodides with alpha,beta-unsaturated esters, nitriles, and ketones proceeds in moderate to excellent yields (50-95%) using Ni(OAc)(2) (0.05-0.2 equiv)-BER (3-5 equiv) in methanol in 1-9 h at room temperature or at 65 degrees C. Nickel boride on borohydride exchange resin (BER) is a good alternative reagent to tributyltin hydride for the coupling of alkyl iodides with the electron
-
PROCESSES FOR PREPARING 2-ISOPROPENYL-5-METHYL-4-HEXENOIC ACID, 2-ISOPROPENYL-5-METHYL-4-HEXEN-1-OL, AND A CARBOXYLATE ESTER THEREOF申请人:Shin-Etsu Chemical Co., Ltd.公开号:US20210323902A1公开(公告)日:2021-10-21The present invention provides a process for preparing 2-isopropenyl-5-methyl-4-hexenoic acid of the following formula (4), comprising steps of: subjecting a Grignard reagent of the following general formula (1), wherein R 1 represents a linear, branched, or aromatic monovalent hydrocarbon group having 1 to 8 carbon atoms, and X represents a chlorine atom, a bromine atom, or an iodine atom, and 1,1,1,3,3,3-hexamethyldisilazane to a deprotonation reaction to form a 1,1,1,3,3,3-hexamethyldisilazane derivative; and subjecting 2-methyl-3-buten-2-yl 3-methyl-2-butenoate of the following formula (3) to a rearrangement reaction in the presence of the 1, 1, 1,3,3,3-hexamethyldisilazane derivative to form 2-isopropenyl-5-methyl-4-hexenoic acid (4).
表征谱图
-
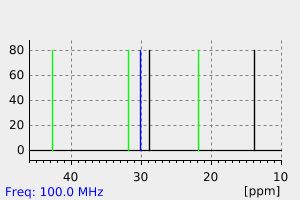
氢谱1HNMR
-
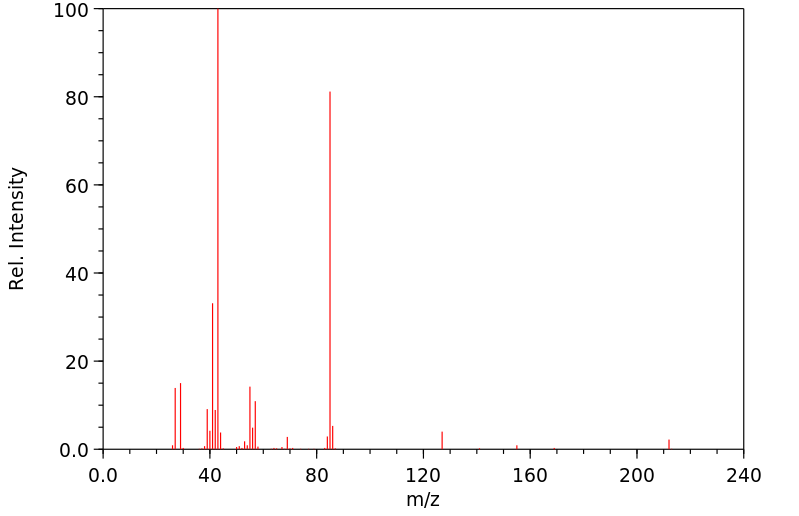
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物