3-溴-5-氟三氟甲苯 | 130723-13-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:138-139 °C (lit.)
-
密度:1.511 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解,未有已知危险反应。请避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:4
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S23,S24/25,S26,S36
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2903999090
-
危险类别:IRRITANT
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:请将贮藏器密封保存,并存放在阴凉、干燥处。同时,确保工作环境具有良好的通风或排气设施。
SDS
模块 1. 化学品
1.1 产品标识符
: 3-溴-5-氟三氟甲苯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C7H3BrF4
分子式
: 243.00 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 溴化氢气, 氟化氢
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
打开了的容器必须仔细重新封口并保持竖放位置以防止泄漏。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要对呼吸系统保护.对少量挥发请采用美国OV/AG (US)标准类型的 或欧洲ABEK (EU EN
14387)标准类型的呼吸器过滤器.
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 液体
颜色: 无色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
无数据资料
f) 沸点、初沸点和沸程
138 - 139 °C - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
1.511 g/cm3 在 25 °C
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
3-溴-5-氟三氟甲苯在常温常压下呈现无色或浅黄色液体的状态。其苯环上的溴单元可以通过Suzuki偶联反应连接一个芳基;也可以将溴转换为硼单元,进一步通过多种硼化学转化来衍生化该化合物。
用途3-溴-5-氟三氟甲苯是一种烃类衍生物,广泛用作药物分子和有机合成的中间体。
合成方法首先,将苯胺的前体化合物与6M HCl 10 mL 放入200 mL 烧杯中,并加热5分钟(使用热风枪)。然后,在-5 °C 下,向反应混合物中滴加NaNO2 (393 mg, 5.7 mmol) 在3 mL 水中的溶液。将所得澄清溶液小心倒入含有NaI(2.43 g,16.2 mmol)溶解在20 mL 水中的溶液中,并在室温下搅拌反应混合物10小时。用CH2Cl2 萃取反应混合物,然后依次用10% NaHSO3 溶液、2M NaOH 溶液和盐水洗涤合并的有机层。最后,使用Na2SO4 干燥并浓缩合并后的有机层。
反应信息
-
作为反应物:描述:3-溴-5-氟三氟甲苯 在 tris-(dibenzylideneacetone)dipalladium(0) 、 三氟乙酸 、 2-(二叔丁基膦)联苯 、 sodium t-butanolate 作用下, 以 二氯甲烷 、 甲苯 为溶剂, 反应 13.25h, 生成 1-[3-Fluoro-5-(trifluoromethyl)phenyl]-piperazine参考文献:名称:[EN] LPXH TARGETING COMPOUNDS, COMPOSITIONS THEREOF, AND METHODS OF MAKING AND USING THE SAME
[FR] COMPOSÉS DE CIBLAGE LPXH, LEURS COMPOSITIONS ET PROCÉDÉS DE FABRICATION ET D'UTILISATION ASSOCIÉS摘要:LpxH靶向化合物,其组合物,以及制备和使用这些化合物的方法在此披露。LpxH靶向化合物通常具有符合式(I)的结构和/或其盐,其中Rb从单键,C4到C10未取代芳基,C4到C10取代芳基,未取代或取代的四到十元杂环环,C1到C10未取代烷基,和C1到C10取代烷基中选择;Rc包括氢,卤素,-OH,-CO2CH3,-COOH,-CN2CF3,-CF3,-C2OH,-CONHOH,-CCOH,C4到C10未取代芳基,C4到C10取代芳基,未取代或取代的四到十元杂环环,C1到C10未取代烷基,或C1到C10取代烷基;而Rd和Re独立地是氢,-OH,-COH,-COH,-COC,-COOH,Rf,或作为未取代或取代的四到八元含氮杂环环一起取代。公开号:WO2021072369A1 -
作为产物:描述:3-氟-5-溴苯酚 在 bis(1,5-cyclooctadiene)nickel (0) 、 三乙胺 、 三甲基膦 作用下, 以 1,4-二氧六环 、 二氯甲烷 为溶剂, 反应 9.17h, 生成 3-溴-5-氟三氟甲苯参考文献:名称:通过 Ar-O 和 Ar-Cl(Br) 键的化学选择性断裂,镍介导的氯化和溴化苯酚衍生物的发散性三氟甲基化摘要:我们在此报道了镍介导的氯化和溴化苯酚衍生物 ClArOTs 和 BrArOTf 的三氟甲基化通过 Ar-O 键的化学选择性断裂得到氯(溴)三氟甲基芳烃。此外,在相似的反应条件下,ClArOPiv和BrArOTs的Ar-Cl和Ar-Br键发生化学选择性三氟甲基化,得到三氟甲基化苯酚衍生物。DOI:10.1021/acs.orglett.4c03020
文献信息
-
Substituted imidazol-pyridazine derivatives申请人:——公开号:US20030229096A1公开(公告)日:2003-12-11The present invention relates to compounds of formula 1 wherein A is an unsubstituted or substituted cyclic group; and R is hydrogen or lower alkyl; or a pharmaceutically acceptable acid addition salt thereof. These compounds are NMDA NR-2B receptor subtype specific blockers and are useful in the treatment of neurodegeneration, depression and pain.本发明涉及以下式的化合物 1 其中A是未取代或取代的环状基团;以及 R是氢或较低的烷基; 或其药学上可接受的酸盐。这些化合物是NMDA NR-2B受体亚型特异性阻断剂,对于治疗神经退行性疾病、抑郁症和疼痛具有用处。
-
Investigations into the Potential Role of Metabolites on the Anti-Leukemic Activity of Imatinib, Nilotinib and Midostaurin作者:Paul W. ManleyDOI:10.2533/chimia.2019.561日期:——
The efficacy and side-effects of drugs do not just reflect the biochemical and pharmacodynamic properties of the parent compound, but often comprise of cooperative effects between the properties of the parent and active metabolites. Metabolites of imatinib, nilotinib and midostaurin have been synthesised and evaluated in assays to compare their properties as protein kinase inhibitors with the parent drugs. The N-desmethyl-metabolite of imatinib is substantially less active than imatinib as a BCR-ABL1 kinase inhibitor, thus providing an explanation as to why patients producing high levels of this metabolite show a relatively low response rate in chronic myeloid leukaemia (CML) treatment. The hydroxymethylphenyl and N-oxide metabolites of imatinib and nilotinib are only weakly active as BCR-ABL1 inhibitors and are unlikely to play a role in the efficacy of either drug in CML. The 3-(R)-HO-metabolite of midostaurin shows appreciable accumulation following chronic drug administration and, in addition to mutant forms of FLT3, potently inhibits the PDPK1 and VEGFR2 kinases (IC50 values
药物的功效和副作用不仅仅反映了母化合物的生化和药效特性,而且通常包括母化合物和活性代谢物之间的协同效应。已经合成和评估了伊马替尼、尼洛替尼和米多斯他林的代谢物,以比较它们作为蛋白激酶抑制剂的特性与母药的区别。伊马替尼的N-去甲基代谢物作为BCR-ABL1激酶抑制剂的活性明显低于伊马替尼,这解释了为什么产生高水平该代谢物的患者在慢性髓细胞白血病(CML)治疗中显示相对较低的反应率。伊马替尼和尼洛替尼的羟甲基苯和N-氧代谢物作为BCR-ABL1抑制剂的活性很弱,不太可能在CML中发挥作用。米多斯他林的3-(R)-HO-代谢物在长期用药后显示出明显的积累,并且除了FLT3的突变形式外,还强力抑制PDPK1和VEGFR2激酶(IC50值 -
[EN] TETRAHYDROPYRIDOPYRIMIDINES AND TETRAHYDROPYRIDOPYRIDINES AS INHIBITORS OF HBSAG (HBV SURFACE ANTIGEN) AND HBV DNA PRODUCTION FOR THE TREATMENT OF HEPATITIS B VIRUS INFECTIONS<br/>[FR] TÉTRAHYDROPYRIDOPYRIMIDINES ET TÉTRAHYDROPYRIDOPYRIDINES COMME INHIBITEURS D'AG HBS (ANTIGÈNE DE SURFACE DU VIRUS DE L'HÉPATITE B) ET PRODUCTION D'ADN DE VHB POUR LE TRAITEMENT D'INFECTIONS PAR LE VIRUS DE L'HÉPATITE B申请人:HOFFMANN LA ROCHE公开号:WO2016177655A1公开(公告)日:2016-11-10The present invention provides tetrahydropyridopyrimidines and tetrahydropyridopyridines having the general formula (I) wherein R1, R2, U, W, X, Y and Z are as described herein, as inhibitors of HBsAg (HBV surface antigen) and HBV DNA production for the treatment and prophylaxis of hepatitis B virus infections.
-
Process for preparation of benzo[f]quinolinones申请人:Eli Lilly and Company公开号:US05578724A1公开(公告)日:1996-11-26A series of benzoquinolin-3-ones are pharmaceuticals effective in treating conditions consequent on both Type I and Type II 5.alpha.-reductase and their preparation is disclosed.一系列苯醌啉-3-酮类药物在治疗由I型和II型5α-还原酶引起的疾病方面具有有效性,并且其制备方法已被披露。
-
Steroid receptor modulator compounds and methods申请人:Ligand Pharmaceuticals Incorporated公开号:US05696127A1公开(公告)日:1997-12-09Non-steroidal compounds which are high affinity, high selectivity modulators for steroid receptors are disclosed. Also disclosed are pharmaceutical compositions incorporating such compounds, methods for employing the disclosed compounds and compositions for treating patients requiring steroid receptor agonist or antagonist therapy, intermediates useful in the preparation of the compounds and processes for the preparation of the steroid receptor modulator compounds.
表征谱图
-
氢谱1HNMR
-
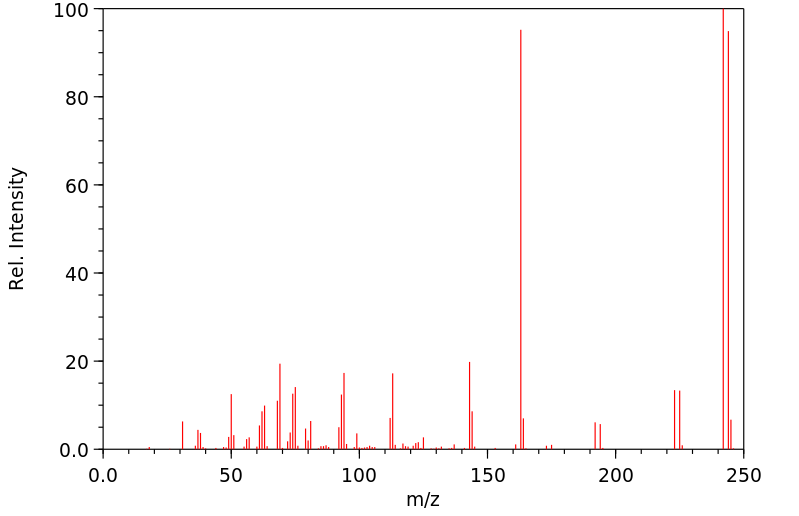
质谱MS
-
碳谱13CNMR
-
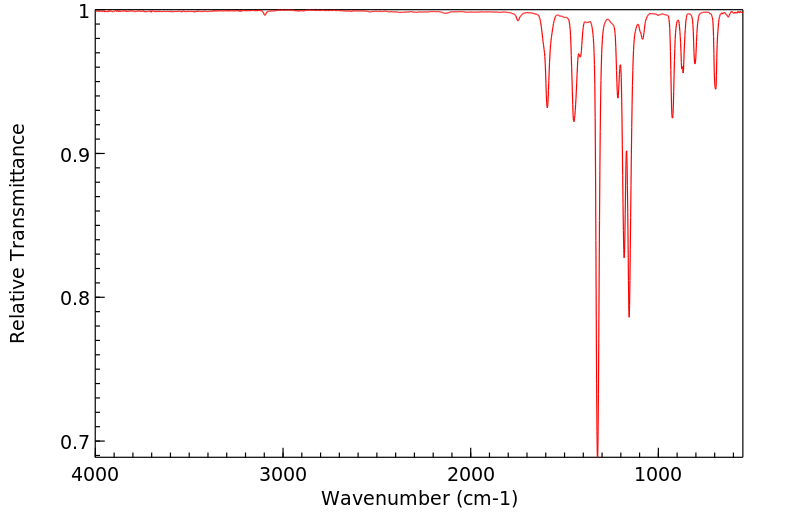
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息