5-甲基吲哚-2-甲酸 | 10241-97-1
中文名称
5-甲基吲哚-2-甲酸
中文别名
5-甲基吲哚-2-羧酸
英文名称
5-methylindole-2-carboxylic acid
英文别名
5-methyl-1H-indole-2-carboxylic acid;5-Methyl-indol-2-carbonsaeure;NSC 88873
CAS
10241-97-1
化学式
C10H9NO2
mdl
MFCD00047166
分子量
175.187
InChiKey
DAITVOCMWPNFTL-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:236-238°C (dec.)
-
沸点:236 °C(Press: 4.0 Torr)
-
密度:1.340±0.06 g/cm3(Predicted)
-
稳定性/保质期:
遵照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:13
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:53.1
-
氢给体数:2
-
氢受体数:2
安全信息
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933990090
-
安全说明:S26,S36
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封于阴凉干燥处。
SDS
Version 1.2
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 5-METHYLINDOLE-2-CARBOXYLIC AC - 250MG
ID
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
5-METHYLINDOLE-2-CARBOXYLIC ACID 10241-97-1 None None
Formula C10H9NO2
Molecular Weight 175,1900 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
ALDRICH www.molbase.com
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear protective equipment.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
PERSONAL PROTECTIVE EQUIPMENT
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range N/A
MP/MP Range 234,000. - 235,000 °
C.
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
ALDRICH www.molbase.com
Stable: Stable.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
ROUTE OF EXPOSURE
Multiple Routes: May cause irritation. May be harmful by
inhalation, ingestion, or skin absorption.
CONDITIONS AGGRAVATED BY EXPOSURE
The toxicological properties have not been thoroughly
investigated.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
ALDRICH www.molbase.com
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
Regulation (EC) No 1907/2006
1 - Product and Company Information
Product Name 5-METHYLINDOLE-2-CARBOXYLIC AC - 250MG
ID
2 - Hazards Identification
3 - Composition/Information on Ingredients
Product Name CAS # EC no Annex I
Index Number
5-METHYLINDOLE-2-CARBOXYLIC ACID 10241-97-1 None None
Formula C10H9NO2
Molecular Weight 175,1900 AMU
4 - First Aid Measures
AFTER INHALATION
If inhaled, remove to fresh air. If breathing becomes difficult,
call a physician.
AFTER SKIN CONTACT
In case of skin contact, flush with copious amounts of water for
at least 15 minutes. Remove contaminated clothing and shoes.
Call a physician.
AFTER EYE CONTACT
In case of contact with eyes, flush with copious amounts of
water for at least 15 minutes. Assure adequate flushing by
separating the eyelids with fingers. Call a physician.
AFTER INGESTION
If swallowed, wash out mouth with water provided person is
conscious. Call a physician.
5 - Fire Fighting Measures
EXTINGUISHING MEDIA
ALDRICH www.molbase.com
Suitable: Water spray. Carbon dioxide, dry chemical powder, or
appropriate foam.
SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS
Wear self-contained breathing apparatus and protective clothing
to prevent contact with skin and eyes.
6 - Accidental Release Measures
PROCEDURE(S) OF PERSONAL PRECAUTION(S)
Wear protective equipment.
METHODS FOR CLEANING UP
Sweep up, place in a bag and hold for waste disposal. Avoid
raising dust. Ventilate area and wash spill site after material
pickup is complete.
7 - Handling and Storage
8 - Exposure Controls / Personal Protection
ENGINEERING CONTROLS
Mechanical exhaust required.
PERSONAL PROTECTIVE EQUIPMENT
Special Protective Measures: Wear appropriate government approved
respirator, chemical-resistant gloves, safety goggles, other
protective clothing.
9 - Physical and Chemical Properties
pH N/A
BP/BP Range N/A
MP/MP Range 234,000. - 235,000 °
C.
Flash Point N/A
Flammability N/A
Autoignition Temp N/A
Oxidizing Properties N/A
Explosive Properties N/A
Explosion Limits N/A
Vapor Pressure N/A
Partition Coefficient N/A
Viscosity N/A
Vapor Density N/A
Saturated Vapor Conc. N/A
Evaporation Rate N/A
Bulk Density N/A
Decomposition Temp. N/A
Solvent Content N/A
Water Content N/A
Surface Tension N/A
Conductivity N/A
Miscellaneous Data N/A
Solubility N/A
10 - Stability and Reactivity
STABILITY
ALDRICH www.molbase.com
Stable: Stable.
HAZARDOUS POLYMERIZATION
Hazardous Polymerization: Will not occur
11 - Toxicological Information
ROUTE OF EXPOSURE
Multiple Routes: May cause irritation. May be harmful by
inhalation, ingestion, or skin absorption.
CONDITIONS AGGRAVATED BY EXPOSURE
The toxicological properties have not been thoroughly
investigated.
12 - Ecological Information
No data available.
13 - Disposal Considerations
SUBSTANCE DISPOSAL
Dissolve or mix the material with a combustible solvent and burn
in a chemical incinerator equipped with an afterburner and
scrubber. Observe all federal, state, and local environmental
regulations.
14 - Transport Information
RID/ADR
Non-hazardous for road transport.
IMDG
Non-hazardous for sea transport.
IATA
Non-hazardous for air transport.
15 - Regulatory Information
Caution: Substance not yet fully tested (EU).
COUNTRY SPECIFIC INFORMATION
Germany
WGK: 3
Self-Classification
16 - Other Information
WARRANTY
The above information is believed to be correct but does not
purport to be all inclusive and shall be used only as a guide. The
information in this document is based on the present state of our
knowledge and is applicable to the product with regard to
appropriate safety precautions. It does not represent any
guarantee of the properties of the product. Inc.,
shall not be held liable for any damage resulting from handling or
from contact with the above product. See reverse side of invoice
or packing slip for additional terms and conditions of sale.
ALDRICH www.molbase.com
Copyright 2010 Co. License granted to make
unlimitedpaper copies for internal use only.
DISCLAIMER
For R&D use only. Not for drug, household or other uses.
ALDRICH www.molbase.com
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-甲基-1H-吲哚-2-羧酸甲酯 methyl 5-methyl-1H-indole-2-carboxylate 102870-03-1 C11H11NO2 189.214 5-甲基吲哚-2-甲基酸酯 5-methyl-1H-indole-2-carboxylic acid ethyl ester 16382-15-3 C12H13NO2 203.241 5-甲基-1H-吲哚-2-甲醛 5-methyl-1H-indole-2-carbaldehyde 1463-60-1 C10H9NO 159.188 —— (5-methyl-1H-indol-2-yl)methanol 55795-87-4 C10H11NO 161.203 1,5-二甲基-1H-吲哚-2-羧酸 1,5-dimethyl-1H-indole-2-carboxylic acid 216210-59-2 C11H11NO2 189.214 5-甲基-1H-吲哚-2-甲酰氯 5-methyl-1H-indole-2-carbonyl chloride 95538-31-1 C10H8ClNO 193.633 —— 5-methyl-2-acetylindole 89671-81-8 C11H11NO 173.214 —— methyl 1,5-dimethyl-1H-indole-2-carboxylate 167478-82-2 C12H13NO2 203.241 —— 2-(5-Methyl-indol)carbohydrazin 1463-64-5 C10H11N3O 189.217 1,5-二甲基-1H-吲哚-2-甲醛 1,5-dimethyl-1H-indole-2-carbaldehyde 883526-76-9 C11H11NO 173.214 —— (1,5-dimethyl-1H-indol-2-yl)methanol 943117-52-0 C11H13NO 175.23 —— 5-methyl-1H-indole-2-carbonitrile —— C10H8N2 156.187 —— (5-methyl-1H-indol-2-yl)(phenyl)methanone —— C16H13NO 235.285 —— (1-Ethyl-5-methylindol-2-yl)methanol 943109-75-9 C12H15NO 189.257 —— 5-methyl-N-phenyl-1H-indole-2-carboxamide —— C16H14N2O 250.3 —— N-methyl-N-propargyl-5-methyl-1H-indole-2-carboxamide 1250829-38-9 C14H14N2O 226.278 —— 1-(5-methyl-1H-indol-2-yl)-2-(pyridin-3-yl)-ethan-1-one 1278517-82-0 C16H14N2O 250.3 —— 3-((4-chlorophenyl)thio)-5-methyl-1H-indole-2-carboxylic acid 1429473-86-8 C16H12ClNO2S 317.796 5-甲基吲哚 5-methyl-1H-indole 614-96-0 C9H9N 131.177 —— 5-methyl-2-(pyrrolidine-1-carbonyl)-1H-indole —— C14H16N2O 228.294 - 1
- 2
反应信息
-
作为反应物:描述:5-甲基吲哚-2-甲酸 在 草酰氯 、 sodium hydride 作用下, 以 二氯甲烷 、 N,N-二甲基甲酰胺 、 mineral oil 为溶剂, 反应 26.25h, 生成 phenyl 3-((4-chlorophenyl)thio)-5-methyl-1H-indole-2-carboxylate参考文献:名称:Design of Potent Poxvirus Inhibitors of the Heterodimeric Processivity Factor Required for Viral Replication摘要:Smallpox constitutes a major bioterrorism threat, which underscores the need to develop antiviral drugs for rapid response in the event of an attack. Viral processivity factors are attractive drug targets in being both specific and essential for their cognate DNA polymerases to synthesize extended strands of DNA. An in silico model of the vacinnia virus processivity factor, comprised of the A20 and D4 heterocomplex, was constructed and used for lead optimization of an indole-based scaffold identified earlier from a high-throughput screening. On the basis of this model, a new class of potent antivirals against vaccinia virus was designed and synthesized, of which two (24a and 24b) exhibited superior improvement over the parent scaffold (IC50 = 42 and 46 vs 82000 nM, respectively). The ability of 24a to suppress vaccinia DNA synthesis is supported by the inhibition of late viral gene expression, as well as by the diminished incorporation of bromodeoxyuridine into viral replication factories.DOI:10.1021/jm301735k
-
作为产物:描述:(E)-ethyl 2-(p-tolylhydrazono)propanoate 在 zinc(II) chloride 作用下, 生成 5-甲基吲哚-2-甲酸参考文献:名称:Raschen, Justus Liebigs Annalen der Chemie, 1887, vol. 239, p. 227摘要:DOI:
文献信息
-
Copper-Mediated Cascade C–H/N–H Annulation of Indolocarboxamides with Arynes: Construction of Tetracyclic Indoloquinoline Alkaloids作者:Ting-Yu Zhang、Chang Liu、Chao Chen、Jian-Xin Liu、Heng-Ye Xiang、Wei Jiang、Tong-Mei Ding、Shu-Yu ZhangDOI:10.1021/acs.orglett.7b03580日期:2018.1.5An efficient and environmentally benign Cu-mediated method was developed for direct cascade C–H/N–H annulation to construct polyheterocyclic indoloquinoline scaffolds. This method highlights an emerging strategy for transforming inert C–H bonds into versatile functional groups in organic synthesis and provides a new versatile approach for the efficient synthesis of indolo[3,2-c] and [2,3-c]quinoline
-
Diamine derivatives申请人:Ohta Toshiharu公开号:US20050020645A1公开(公告)日:2005-01-27A compound represented by the general formula (1): Q 1 -Q 2 -T 0 -N(R 1 )-Q 3 -N(R 2 )-T 1 -Q 4 (1) wherein R 1 and R 2 are hydrogen atoms or the like; Q 1 is a saturated or unsaturated, 5- or 6-membered cyclic hydrocarbon group which may be substituted, or the like; Q 2 is a single bond or the like; Q 3 is a group in which Q 5 is an alkylene group having 1 to 8 carbon atoms, or the like; and T 0 and T 1 are carbonyl groups or the like; a salt thereof, a solvate thereof, or an N-oxide thereof. The compound is useful as an agent for preventing and/or treating cerebral infarction, cerebral embolism, myocardial infarction, angina pectoris, pulmonary infarction, pulmonary embolism, Buerger's disease, deep venous thrombosis, disseminated intravascular coagulation syndrome, thrombus formation after valve or joint replacement, thrombus formation and reocclusion after angioplasty, systemic inflammatory response syndrome (SIRS), multiple organ dysfunction syndrome (MODS), thrombus formation during extracorporeal circulation, or blood clotting upon blood drawing.通用式(1)表示的化合物: Q1-Q2-T0-N(R1)-Q3-N(R2)-T1-Q4(1) 其中R1和R2是氢原子或类似物;Q1是饱和或不饱和的、5-或6-成员环烃基,可以被取代,或类似物;Q2是单键或类似物;Q3是一个基团,其中Q5是具有1至8个碳原子的烷基基团,或类似物;T0和T1是羰基团或类似物;其盐、溶剂合物或N-氧化物。 该化合物可用作预防和/或治疗脑梗死、脑栓塞、心肌梗死、心绞痛、肺梗死、肺栓塞、布尔格病、深静脉血栓形成、弥散性血管内凝血综合征、瓣膜或关节置换后的血栓形成、血管成形术后的血栓形成和再闭塞、全身性炎症反应综合征(SIRS)、多器官功能障碍综合征(MODS)、体外循环期间的血栓形成,或抽血时的血液凝结。
-
Identification and Optimization of Pyrrolidine Derivatives as Highly Potent Ghrelin Receptor Full Agonists作者:Martin Cooper、Antonio Llinas、Peter Hansen、Moya Caffrey、Asim Ray、Stina Sjödin、Igor Shamovsky、Hiroki Wada、Tina Jellesmark Jensen、Ulf Sivars、Leif Hultin、Ulf Andersson、Sara Lundqvist、Karin Gedda、Lisa Jinton、Nina Krutrök、Richard Lewis、Paul Jansson、Cristina GardelliDOI:10.1021/acs.jmedchem.0c00828日期:2020.9.10adipogenesis and therefore has been considered a promising therapeutic target for catabolic conditions. We previously reported on the synthesis and properties of an indane based series of ghrelin receptor full agonists which led to a sustained increase of insulin-like growth factor-1 in a dog pharmacodynamic study. Herein we report on the identification of a series of pyrrolidine or piperidine based
-
[EN] SUBSTITUTED TRICYCLIC AMIDES, ANALOGUES THEREOF, AND METHODS USING SAME<br/>[FR] AMIDES TRICYCLIQUES SUBSTITUÉS, ANALOGUES DE CEUX-CI ET PROCÉDÉS LES METTANT EN OEUVRE申请人:ARBUTUS BIOPHARMA CORP公开号:WO2021229302A1公开(公告)日:2021-11-18The present disclosure includes substituted tricyclic amides, or analogues thereof of formula (I) (I), wherein X, Y, ring A, R1, R5, R6 and R7 are as defined herein, and compositions comprising compounds of formula (I) that can be used to treat or prevent hepatitis B virus (HBV) and/or hepatitis D virus (HDV) infections in a patient.
-
Novel flavors, flavor modifiers, tastants, taste enhancers, umami or sweet tastants, and/or enhancers and use thereof申请人:Tachdjian Catherine公开号:US20050084506A1公开(公告)日:2005-04-21The present invention relates to the discovery that certain non-naturally occurring, non-peptide amide compounds and amide derivatives, such as oxalamides, ureas, and acrylamides, are useful flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancer, more particularly, savory (the “umami” taste of monosodium glutamate) or sweet taste modifiers,—savory or sweet flavoring agents and savory or sweet flavor enhancers, for food, beverages, and other comestible or orally administered medicinal products or compositions.
表征谱图
-
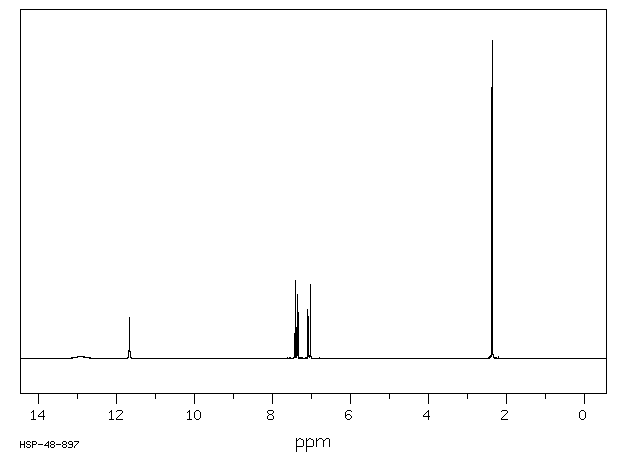
氢谱1HNMR
-
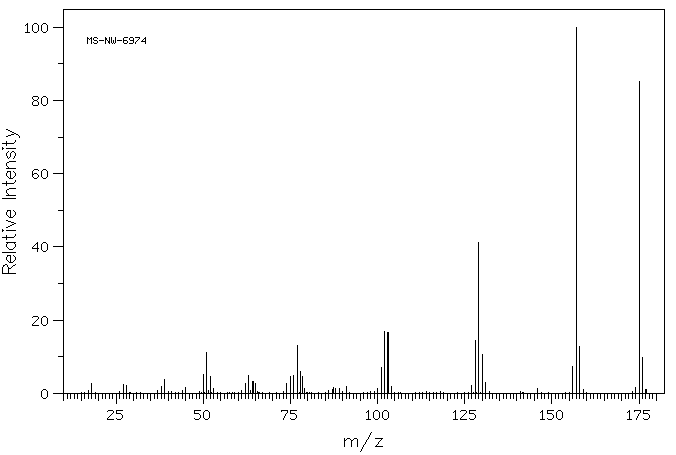
质谱MS
-
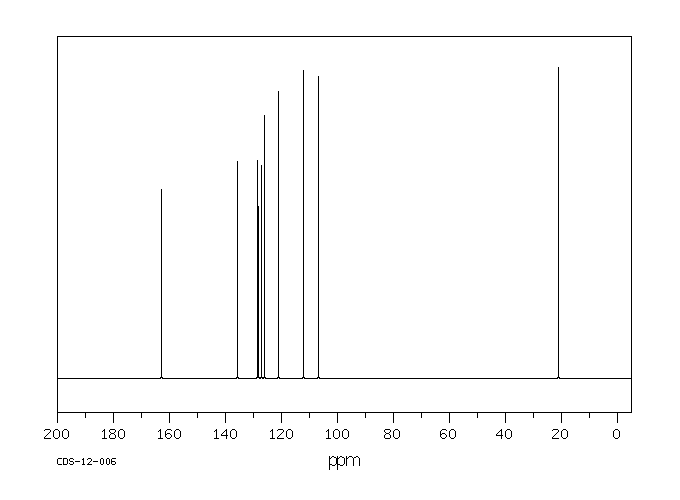
碳谱13CNMR
-
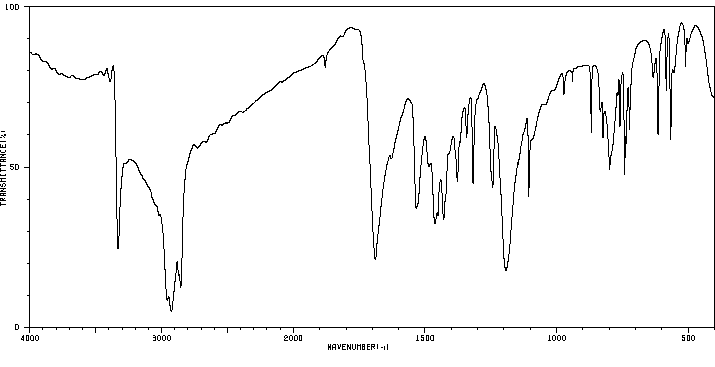
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3