4-二甲基氨基-2-甲氧基苯甲醛 | 84562-48-1
中文名称
4-二甲基氨基-2-甲氧基苯甲醛
中文别名
4-二甲氨基-2-甲氧基苯甲醛;4-二甲胺基-2-甲氧基苯甲醛
英文名称
4-dimethylamino-2-methoxybenzaldehyde
英文别名
2-methoxy-4-(dimethylamino)benzaldehyde;4-(dimethylamino)-2-methoxybenzaldehyde
CAS
84562-48-1
化学式
C10H13NO2
mdl
MFCD00151814
分子量
179.219
InChiKey
HGDRXADJVGVGBC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:58-60°C
-
沸点:150-152 °C(Press: 0.1 Torr)
-
密度:1.098±0.06 g/cm3(Predicted)
-
溶解度:25℃时微溶0.32g/L。
-
稳定性/保质期:
按规定使用和贮存时不会分解,并应避免与氧化物、空气接触。
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:13
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:29.5
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36
-
危险类别码:R36/37/38
-
海关编码:2922509090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:请将药品存放在密闭、阴凉、干燥的地方。
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(二甲氨基)邻羟苄基 4-dimethylamino-2-hydroxy-benzaldehyde 41602-56-6 C9H11NO2 165.192 3-二甲基氨基苯甲醚 3-methoxy-N,N-dimethylaniline 15799-79-8 C9H13NO 151.208
反应信息
-
作为反应物:参考文献:名称:Discovery of 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7-phenyl-(E)-2,3,6,7-tetrahydro-1,4-thiazepines as a new series of apoptosis inducers using a cell- and caspase-based HTS assay摘要:We report the discovery of 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7-(4-methylphenyl)-(E)-2,3,6,7-tetrahydro-1,4-thiazepine (2a) as an inducer of apoptosis using our proprietary cell- and caspase-based HTS assay. Through structure activity relationship (SAR) studies, 5-(4-hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-7-(2-methoxy-4-(methylthio)phenyl)-(E)-2,3,6,7-tetrahydro-1,4-thiazepine (5d) was identified as a potent apoptosis inducer with an EC50 value of 0.08 mu M in T47D cells, which was > 15-fold more potent than screening hit 2a. Compound 5d also was found to be highly active in a growth inhibition assay with a GI(50) value of 0.05 mu M in T47D cells and to function as an inhibitor of tubulin polymerization. (c) 2007 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmcl.2007.05.098
-
作为产物:描述:溴甲烷 、 4-(二甲氨基)邻羟苄基 在 potassium carbonate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 8.0h, 以68.1%的产率得到4-二甲基氨基-2-甲氧基苯甲醛参考文献:名称:Effect of phenyl ring substitution on J-aggregate formation ability of novel bisazomethine dyes in vapour-deposited films摘要:Bisazomethine dyes, which are synthesized using diaminomaleonitrile and aminobenzaldehydes, exhibit red fluorescence in solution and in the solid state. Several bisazomethine dyes are known to form J-aggregates in vapour-deposited films. In this work, novel bisazomethine dyes were synthesized and the effect of phenyl ring substitution on the J-aggregate formation in vapour-deposited films was examined. The optical properties of the dyes were examined in solution and in the solid state through molecular orbital calculations. Four derivatives were found to form J-aggregates in vapour-deposited films as determined from the shape of the spectrum and the absorption edge. (C) 2010 Elsevier Ltd. All rights reserved.DOI:10.1016/j.dyepig.2010.11.009
文献信息
-
Novel Thiophene Derivatives as Spingosine-1-Phosphate-1 Receptor Agonists
-
[EN] USE OF AND SOME NOVEL IMIDAZOPYRIDINES<br/>[FR] NOUVELLES IMIDAZOPYRIDINES ET LEUR UTILISATION申请人:ASTRAZENECA AB公开号:WO2004016611A1公开(公告)日:2004-02-26The use of compounds of formula (I) wherein R1, R3, R10, m and Ar are as defined in the Specification and pharmaceutically acceptable salts thereof in the manufacture of a medicament for the treatment or prophylaxis of diseases or conditions in which inhibition of kinase Itk activity is beneficial is disclosed. Certain novel compounds of formula (I), together with processes for their preparation, compositions containing them and their use in therapy are also disclosed.公开了在制备药物用于治疗或预防抑制激酶Itk活性有益的疾病或症状中,使用式(I)中R1、R3、R10、m和Ar所定义的化合物及其药学上可接受的盐。还公开了式(I)的某些新化合物,以及它们的制备方法、含有它们的组合物和它们在治疗中的用途。
-
Cobalt-catalysed C–H methylation for late-stage drug diversification作者:Stig D. Friis、Magnus J. Johansson、Lutz AckermannDOI:10.1038/s41557-020-0475-7日期:2020.6despite its significance, accessing such analogues via derivatization at a late stage remains a pivotal challenge. In an effort to mitigate this major limitation, we here present a strategy for the cobalt-catalysed late-stage C–H methylation of structurally complex drug molecules. Enabling broad applicability, the transformation relies on a boron-based methyl source and takes advantage of inherently present
-
Monofluoroalkenylation of Dimethylamino Compounds through Radical–Radical Cross‐Coupling作者:Jin Xie、Jintao Yu、Matthias Rudolph、Frank Rominger、A. Stephen K. HashmiDOI:10.1002/anie.201602347日期:2016.8An unprecedented and challenging radical–radical cross‐coupling of α‐aminoalkyl radicals with monofluoroalkenyl radicals derived from gem‐difluoroalkenes was achieved. This first example of tandem C(sp3)−H and C(sp2)−F bond functionalization through visible‐light photoredox catalysis offers a facile and flexible access to privileged tetrasubstituted monofluoroalkenes under very mild reaction conditions
-
Improved PeT Molecules for Optically Sensing Voltage in Neurons作者:Clifford R. Woodford、E. Paxon Frady、Richard S. Smith、Benjamin Morey、Gabriele Canzi、Sakina F. Palida、Ricardo C. Araneda、William B. Kristan、Clifford P. Kubiak、Evan W. Miller、Roger Y. TsienDOI:10.1021/ja510602z日期:2015.2.11VoltageFluor (VF) dyes have the potential to measure voltage optically in excitable membranes with a combination of high spatial and temporal resolution essential to better characterize the voltage dynamics of large groups of excitable cells. VF dyes sense voltage with high speed and sensitivity using photoinduced electron transfer (PeT) through a conjugated molecular wire. We show that tuning theVoltageFluor (VF) 染料具有在可兴奋膜中光学测量电压的潜力,结合高空间和时间分辨率,对于更好地表征大群可兴奋细胞的电压动态至关重要。VF 染料通过共轭分子线使用光诱导电子转移 (PeT) 以高速和灵敏的方式检测电压。我们表明,通过系统的化学取代调整 PeT (ΔGPeT + w) 的驱动力可以调节电压灵敏度,根据实验测量的氧化还原电位估计 (ΔGPeT + w) 值,并验证膜片钳 HEK 电池中 10 个新 VF 的电压敏感性染料。VF2.1(OMe).H,每 100 mV 的 ΔF/F 为 48%,在 HEK 细胞、分离的大鼠皮质神经元和药用水蛭神经节中显示出比以前的染料提高了大约 2 倍。此外,
表征谱图
-
氢谱1HNMR
-
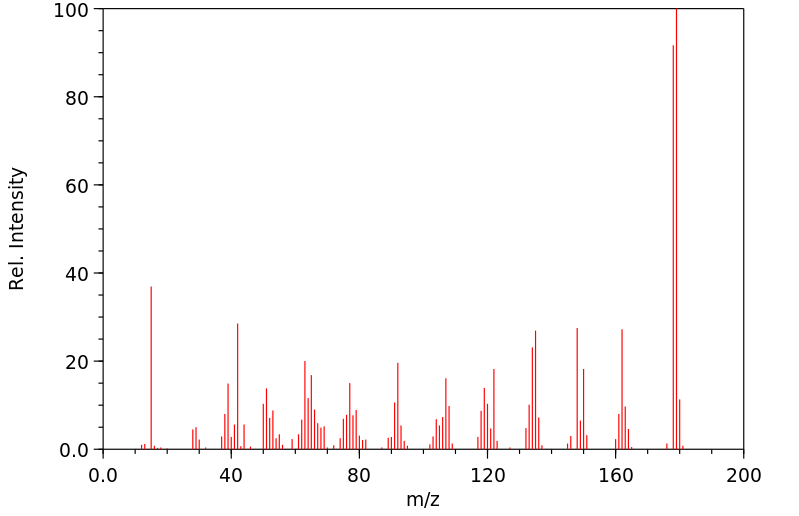
质谱MS
-
碳谱13CNMR
-
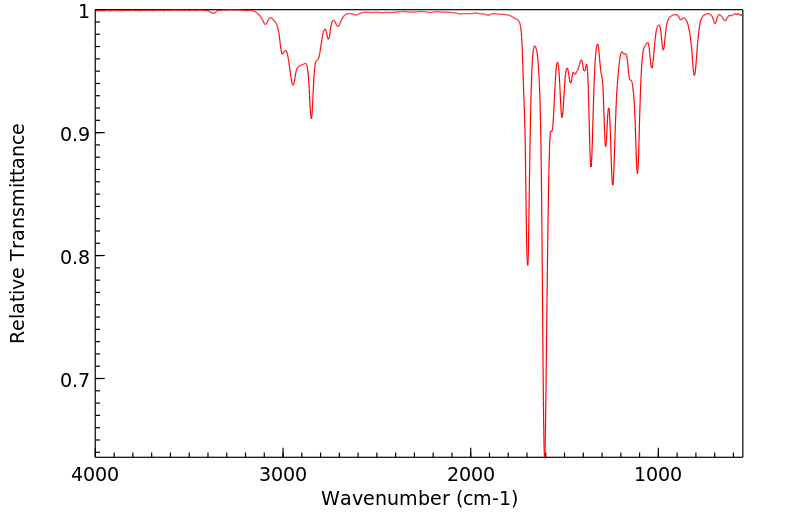
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-(叔丁基)-4-(2,6-二异丙氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(2S,3R)-3-(叔丁基)-2-(二叔丁基膦基)-4-甲氧基-2,3-二氢苯并[d][1,3]氧杂磷杂戊环
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2R,2''R,3R,3''R)-3,3''-二叔丁基-4,4''-二甲氧基-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2-氟-3-异丙氧基苯基)三氟硼酸钾
(+)-6,6'-{[(1R,3R)-1,3-二甲基-1,3基]双(氧)}双[4,8-双(叔丁基)-2,10-二甲氧基-丙二醇
麦角甾烷-6-酮,2,3,22,23-四羟基-,(2a,3a,5a,22S,23S)-
鲁前列醇
顺式6-(对甲氧基苯基)-5-己烯酸
顺式-铂戊脒碘化物
顺式-四氢-2-苯氧基-N,N,N-三甲基-2H-吡喃-3-铵碘化物
顺式-4-甲氧基苯基1-丙烯基醚
顺式-2,4,5-三甲氧基-1-丙烯基苯
顺式-1,3-二甲基-4-苯基-2-氮杂环丁酮
非那西丁杂质7
非那西丁杂质3
非那西丁杂质22
非那西丁杂质18
非那卡因
非布司他杂质37
非布司他杂质30
非布丙醇
雷诺嗪
阿达洛尔
阿达洛尔
阿莫噁酮
阿莫兰特
阿维西利
阿索卡诺
阿米维林
阿立酮
阿曲汀中间体3
阿普洛尔
阿普斯特杂质67
阿普斯特中间体
阿普斯特中间体
阿托西汀EP杂质A
阿托莫西汀杂质24
阿托莫西汀杂质10
阿托莫西汀EP杂质C
阿尼扎芬
阿利克仑中间体3
间苯胺氢氟乙酰氯
间苯二酚二缩水甘油醚
间苯二酚二异丙醇醚
间苯二酚二(2-羟乙基)醚
间苄氧基苯乙醇
间甲苯氧基乙酸肼
间甲苯氧基乙腈
间甲苯异氰酸酯