环丙基碘 | 19451-11-7
中文名称
环丙基碘
中文别名
碘环丙烷
英文名称
cyclopropyliodide
英文别名
iodocyclopropane
CAS
19451-11-7
化学式
C3H5I
mdl
MFCD20621267
分子量
167.977
InChiKey
VLODBNNWEWTQJX-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:132-134 °C
-
沸点:92-94 °C
-
密度:2.13±0.1 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:4
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903890090
-
包装等级:III
-
危险类别:3
-
危险性防范说明:P210,P240,P241,P242,P243,P261,P264,P271,P280,P302+P352,P303+P361+P353,P304+P340,P305+P351+P338,P312,P332+P313,P337+P313,P362,P370+P378,P403+P233,P403+P235,P405,P501
-
危险品运输编号:1993
-
危险性描述:H225,H315,H319,H335
-
储存条件:存储条件:2-8°C,密封保存,置于干燥处,避光,使用惰性气体保护。
SDS
反应信息
-
作为反应物:参考文献:名称:Efforts toward the synthesis of liphatic iodonium salts摘要:DOI:10.1021/jo01267a073
-
作为产物:描述:参考文献:名称:Efforts toward the synthesis of liphatic iodonium salts摘要:DOI:10.1021/jo01267a073
文献信息
-
Micelle enabled C(sp<sup>2</sup>)–C(sp<sup>3</sup>) cross-electrophile coupling in water <i>via</i> synergistic nickel and copper catalysis作者:Ning Ye、Bin Wu、Kangming Zhao、Xiaobin Ge、Yu Zheng、Xiaodong Shen、Lei Shi、Margery Cortes-Clerget、Morgan Louis Regnier、Michael Parmentier、Fabrice GallouDOI:10.1039/d1cc02885e日期:——
A robust and sustainable C(sp2)–C(sp3) cross-electrophile coupling was developed
via nickel/copper synergistic catalysis under micellar conditions. -
Visible-Light-Initiated Manganese Catalysis for C−H Alkylation of Heteroarenes: Applications and Mechanistic Studies作者:Philippe Nuhant、Martins S. Oderinde、Julien Genovino、Antoine Juneau、Yohann Gagné、Christophe Allais、Gary M. Chinigo、Chulho Choi、Neal W. Sach、Louise Bernier、Yvette M. Fobian、Mark W. Bundesmann、Bhagyashree Khunte、Mathieu Frenette、Olugbeminiyi O. FadeyiDOI:10.1002/anie.201707958日期:2017.11.27A visible‐light‐driven Minisci protocol that employs an inexpensive earth‐abundant metal catalyst, decacarbonyldimanganese Mn2(CO)10, to generate alkyl radicals from alkyl iodides has been developed. This Minisci protocol is compatible with a wide array of sensitive functional groups, including oxetanes, sugar moieties, azetidines, tert‐butyl carbamates (Boc‐group), cyclobutanes, and spirocycles. The
-
Pyrido[2,3-<i>d</i>]pyrimidin-7-one Inhibitors of Cyclin-Dependent Kinases作者:Mark Barvian、Diane H. Boschelli、Jennifer Cossrow、Ellen Dobrusin、Ali Fattaey、Alex Fritsch、David Fry、Patricia Harvey、Paul Keller、Michelle Garrett、Frances La、Wilbur Leopold、Dennis McNamara、Maire Quin、Susanne Trumpp-Kallmeyer、Peter Toogood、Zhipei Wu、Erli ZhangDOI:10.1021/jm000271k日期:2000.11.1The identification of 8-ethyl-2-phenylamino-8H-pyrido[2, 3-d]pyrimidin-7-one (1) as an inhibitor of Cdk4 led to the initiation of a program to evaluate related pyrido[2, 3-d]pyrimidin-7-ones for inhibition of cyclin-dependent kinases (Cdks). Analysis of more than 60 analogues has identified some clear SAR trends that may be exploited in the design of more potent Cdk inhibitors. The most potent Cdk4鉴定为Cdk4抑制剂的8-乙基-2-苯基氨基-8H-吡啶并[2,3-d]嘧啶-7-一(1)引发了评估相关吡啶并[2,3- d]嘧啶-7-抑制细胞周期蛋白依赖性激酶(Cdks)的化合物。对60多个类似物的分析已经确定了一些明显的SAR趋势,可在设计更有效的Cdk抑制剂时加以利用。本研究中报告的最有效的Cdk4抑制剂以IC(50)= 0.004 microM([ATP] = 25 microM)抑制Cdk4。对与相关激酶Cdk2结合的代表性化合物的X射线晶体学分析表明,它们占据了ATP结合位点。一些化合物在Cdk之间表现出适度的选择性,而Cdk4选择性抑制剂在细胞分裂周期的G(1)相中阻断pRb(+)细胞。
-
C–H Functionalization of Heteroarenes Using Unactivated Alkyl Halides through Visible-Light Photoredox Catalysis under Basic Conditions作者:Noah B. Bissonnette、Michael J. Boyd、Gregory D. May、Simon Giroux、Philippe NuhantDOI:10.1021/acs.joc.8b01589日期:2018.9.21C–H functionalization of electron-deficient heteroarenes using commercial unactivated alkyl halides through reductive quenching photoredox catalysis was developed. Mainstream approaches rely on the use of an excess of strong acids that result in regioselectivities dictated by the innate effect of the protonated heteroarene, leaving the functionalization of other carbons unexplored. We report a mild
-
Engaging 1,7-diynes in a photocatalytic Kharasch-type addition/1,5-(S<sub>N</sub>′′)-substitution cascade toward β-gem-dihalovinyl carbonyls作者:Dan Wu、Wen-Juan Hao、Qian Rao、Yi Lu、Shu-Jiang Tu、Bo JiangDOI:10.1039/d0cc07880h日期:——β-gem-dihalovinyl ketones/aldehydes with moderate to excellent yields in a highly regioselective manner. This reaction tolerates a wide scope of substrates, which offers a green and efficient entry to fabricate synthetically important β-gem-dihalovinyl carbonyl scaffolds. Notably, the late-stage application of these resulting β-gem-dihalovinyl carbonyls shows high and unique reactivity profiles and demonstrates一种新的和一般的光催化Kharasch型加成/ 1,5-(S Ñ '')3'-取代级联的1,7-二炔与烷基卤化物如BrCCl 3和CBR 4报道了第一次,并用于产生迄今未报道的65种β-gem-dihalovinyl酮/醛,具有很高的区域选择性,产率中等至优异。该反应可耐受各种各样的底物,为绿色合成高效的合成重要的β-gem-dihalovinyl羰基支架的制备提供了条件。值得注意的是,这些生成的β-gem-dihalovinyl羰基化合物的后期应用显示出高而独特的反应活性,并证明了其衍生化的多功能性。
表征谱图
-
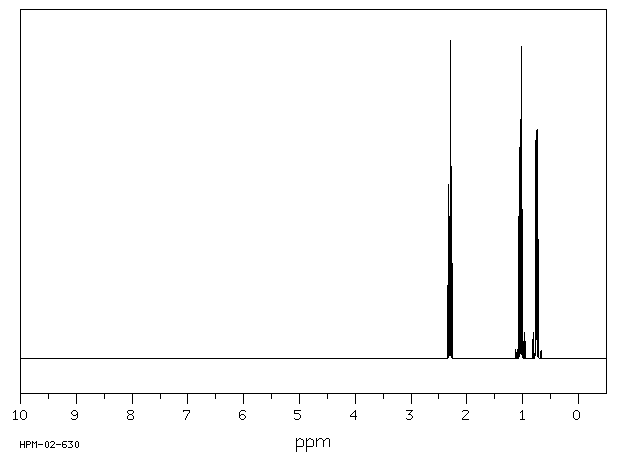
氢谱1HNMR
-
质谱MS
-
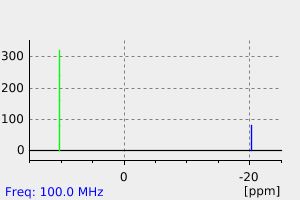
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
胍,N-[3-(氨基甲基)-5-甲基苯基]-N'-乙基-
碘甲烷
碘甲基环辛烷
碘甲基环戊烷
碘环庚烷
碘环十二烷
碘环丁烷
碘十六烷
碘代环戊烷
碘代正辛烷-D2
碘代异丁烷
碘代叔丁烷
碘代丙烷-D7
碘代丙烷-D3
碘代丙烷-D2
碘代丙烷-D2
碘乙烷-d<
碘乙烷-D1
碘乙烷-2-13C
碘乙烷-2,2,2-d3
碘乙烷-1-13C
碘乙烷-1,1-d2
碘乙烷(1,2-13C2)
碘乙烷
碘丁烷-D9
碘(碘甲氧基)甲烷
甲基碘化钙
环辛烷,1-氟-2-碘-,反-
环戊二烯并[1,3]环丙烯并[1,2]环庚烯-2(1H)-酮,八氢-3a,5,5-三甲基-,(3aR,3bR,8aS)-rel-
环丙基碘
无花果蛋白酶来源于无花果树乳胶
新戊氧基
新戊基碘
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
抗-8-碘-1,5-二甲基二环<3.2.1>辛烷
异戊基碘
异丁基锰(II)碘化物
反式-4-己烯基碘
十氢-2-(碘甲基)-萘
十四烷基碘化物
十五氟碘庚烷
十九氟-9-碘壬烷
全氟辛基碘烷
全氟碘代丁烷
全氟异戊基碘
全氟异庚基碘化物
全氟异壬基碘
全氟异十一烷基碘化物
全氟己基碘烷
全氟叔丁基碘化物