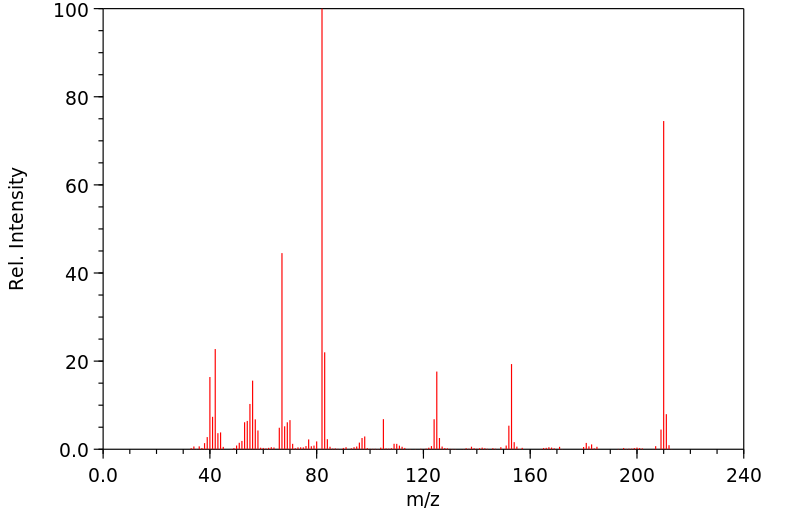
代谢
1,3,7-三甲基尿酸是咖啡因的人类已知代谢物。
1,3,7-Trimethyluric acid is a known human metabolite of caffeine.
来源:NORMAN Suspect List Exchange