1,1'-azobisformamide | 123-77-3
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:220-225 °C (dec.)(lit.)
-
沸点:217.08°C (rough estimate)
-
密度:1.65
-
闪点:225 °C
-
溶解度:水:20°C时可溶0.033g/L
-
LogP:-1.148 (est)
-
物理描述:Azodicarbonamide appears as a yellow to orange powder. Insoluble in water and common solvents. Soluble in dimethyl sulfoxide. Nontoxic.
-
颜色/状态:Orange-red crystals
-
蒸汽压力:1.88X10-10 mm Hg @ 20 °C
-
稳定性/保质期:
Does not react with plasticizers and other components of plastics.
-
分解:/Azoformamide/ shows a high rate of pressure rise on thermal decomposition. Energy of decomposition (in range 160-230 °C) measured as 1.36 kJ/g by /Differential Scanning Calorimeter/, and Tait24 was determined as 120 °C by adiabatic Dewar tests, with an apparent energy of activation of 131 kJ/mol.
计算性质
-
辛醇/水分配系数(LogP):-1
-
重原子数:8
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:111
-
氢给体数:2
-
氢受体数:2
ADMET
安全信息
-
危险等级:4.1
-
危险品标志:Xn
-
安全说明:S22,S24,S37
-
危险类别码:R44,R42
-
WGK Germany:1
-
海关编码:2924199090
-
危险品运输编号:UN 3242 4.1/PG 2
-
危险类别:4.1
-
RTECS号:LQ1040000
-
包装等级:II
SDS
制备方法与用途
取试样35mg,溶于1000ml水中。该溶液的紫外线最大吸收作用在波长245nm处。
含量分析准确称取约225mg(在50℃下真空干燥2h后)的试样,放入一250ml具玻塞碘量瓶中。加入二甲基亚砜约23ml,洗下可能附于壁上的试样,加塞,在塞子周围盛上2ml该溶剂。偶尔转动一下,至试样完全溶解。去掉瓶塞,使剩余的溶剂连同可能溶解的试样流入烧瓶。用15ml水将5.0g碘化钾洗入烧瓶,随后立即吸取0.5mol/L盐酸10ml移入烧瓶,迅速塞盖。摇动瓶子至碘化钾完全溶解,然后在避光条件下静置20~25min,用0.1mol/L硫代硫酸钠滴定至所析出的碘的黄色消失,在15min内如重新出现黄色,则再用硫代硫酸钠滴定至无色。另取25ml二甲基亚砜、5.0g碘化钾、15ml水和5mL0.5mol/L盐酸进行空白试验。每毫升0.1mol/L硫代硫酸钠相当于偶氮甲酰胺(C₂H₄N₄O₂)5.804mg。
毒性可安全用于食品(FDA,§172.806,2000)。 ADI 0~45(FAO/WHO,2001)。
使用限量- GB 2760-1996:小麦粉0.045g/kg。
- FAO/WHO,1965:45mg/kg小麦粉。
- 日本,1999:谷物粉、面包面团,45mg/kg。
黄色粉末。无臭。溶于碱和二甲基亚砜,不溶于醇、汽油、苯、吡啶和水。
用途- 小麦粉处理剂;焙烤食品快速发酵剂。
- 在低用量下可完成对小麦粉的安全快速氧化,以改善面团的物理性能和高筋面团所需的组织结构。
- 作面粉处理剂,我国规定可用于小麦粉中,最大使用量为0.2g/kg(以成品计)。
- 还可用于合成染料、塑料稳定剂等。
将尿素溶于2%的水合肼溶液,搅拌,并加入硫酸,使pH值达到1~2,然后加热使pH值为2~5,再缓缓加入硫酸,保持pH值2~5,反应数小时后取样测定终点;反应结束后,过滤去除硫酸铵母液,并用热水洗涤得联二脲。将联二脲溶于水,加入溴化钠,通入氯气,在30~50℃反应;反应混合物用温水洗至中性,再离心甩干、干燥得成品。
原料消耗定额:水合肼(40%)1160kg/t、尿素(含氮量≥46%)1330kg/t、液氯800kg/t、烧碱(30%)2000kg/t、硫酸(98%)1140kg/t。
类别- 易燃固体
- 毒性分级:低毒
- 急性毒性:口服-大鼠 LD50; >6400 毫克/公斤
- 爆炸物危险特性:低过
- 敏剂
- 可燃性危险特性:易燃;燃烧产生有毒氮氧化物烟雾
- 储运特性:通风低温干燥
反应信息
-
作为反应物:描述:参考文献:名称:POGREBNOJ, A. M.;SOKOLOV, V. N.;KOLESNIKOV, A. A., IZV. VUZOV. XIMIYA I XIM. TEXNOL., 32,(1989) N1, S. 86-91摘要:DOI:
-
作为产物:描述:联二脲 以93%的产率得到参考文献:名称:DRAGUTAN, VALERIAN;DRAGUTAN, ILEANA;VINATORU, MIRCEA;MEDVEDIC, CONSTANTIN+摘要:DOI:
-
作为试剂:描述:邻碘溴苯 、 4-乙炔基苯甲醚 在 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 、 二异丙胺 作用下, 以 1,1'-azobisformamide 为溶剂, 生成 4-(2-bromophenylethynyl)anisole参考文献:名称:Emission from Regioisomeric Bis(phenylethynyl)benzenes during Pulse Radiolysis摘要:Emission from charge recombination between radical cations and anions of a series of regioisomeric 1,4-, 1,3-, and 1,2-bis(phenylethynyl)benzenes (bPEBs) substituted by various electron donor and/or acceptor groups was measured during pulse radiolysis in benzene (Bz). The formation of bPEB in the excited singlet state ((1)bPEB*) can be attributed to the charge recombination between bPEB(center dot+) and bPEB(center dot-), which are initially generated from the radiolytic reaction. This mechanism is reasonably explained by the relationship between the annihilation enthalpy change (-Delta H degrees) for the charge recombination of bPEB(center dot+) and bPEB(center dot-) and excitation energy of (1)bPEB*. Since the degree of the pi-conjugation in the S-1 state and HOMO-LUMO levels of bPEB change with the substitution pattern of phenylacetylene groups on the central benzene ring and the various kinds of donor and/or acceptor group, the fine-tuning of the emission color and intensity of bPEB can be easily carried out during pulse radiolysis in Bz. For donor-acceptor-substituted bPEB, it was found that the difference in the charge transfer conjugated pathways between donor and acceptor substituents (linear-, cross-, and "bent"-conjugated pathways) strongly influenced the HOMO-LUMO energy gap.DOI:10.1021/jo900494j
文献信息
-
Method for producing sulfonyl semicarbazides申请人:Armstrong Cork Company公开号:US03933909A1公开(公告)日:1976-01-20A new synthetic route to sulfonyl semicarbazides wherein an azobisformamide is reacted with a free sulfinic acid.一种新的合成磺酰腙的方法,其中偶氮二甲酰胺与自由亚磺酸反应。
-
2,N-6-disubstituted adenosines and their antihypertensive methods of use申请人:Glaxo Group Limited公开号:US05032583A1公开(公告)日:1991-07-16Compounds of formula (I) ##STR1## wherein X represents a hydrogen or chlorine atom, or a methyl group; and R represents a cycloalkyl or cycloalkenyl ring containing 5 to 8 carbon atoms, which ring is substituted by a hydroxy group, and is optionally substituted by a C.sub.1-6 alkyl group and salts and solvates thereof. The new compounds have been found to exhibit activities such as an anti-lipolytic action. Processes for preparing the compounds of formula (I) and compositions containing them are also described.公式(I)的化合物 其中X代表氢原子、氯原子或甲基基团;R代表含有5至8个碳原子的环状烷基或环状烯基环,该环被羟基取代,并且可以被C.sub.1-6烷基取代,以及其盐和溶剂化物。已发现这些新化合物具有抗脂解作用等活性。本文还描述了制备公式(I)化合物的方法和含有它们的组合物。
-
Divergent Synthesis of Trifluoromethyl-Substituted 1,2-Dihydroquinoxalines and Diimines by Cascade Reactions of CF<sub>3</sub>–Imidoyl Sulfoxonium Ylides with Azo Compounds作者:Guangming Wei、Dongling Zheng、Chen Li、Zhengkai Chen、Xiao-Feng WuDOI:10.1021/acs.orglett.3c02718日期:2023.9.29A base-mediated cascade reaction of CF3-imidoyl sulfoxonium ylides and azo compounds has been achieved, allowing for facile access to trifluoromethyl-substituted 1,2-dihydroquinoxalines and diimines in moderate to excellent yields. Noteworthy is that the unusual N–N bond cleavage and rearrangement of azo compounds are involved in the transformations.
-
Formamoylation of some azo compounds and the characterization of reaction products作者:Richard M. Fantazier、John E. HerwehDOI:10.1021/jo00954a036日期:1973.7
-
1, 2-dicarbamyl-1, 2, 3, 6-tetrahydropyridazines and their preparation申请人:STERLING DRUG INC公开号:US02813867A1公开(公告)日:1957-11-19
表征谱图
-
氢谱1HNMR
-
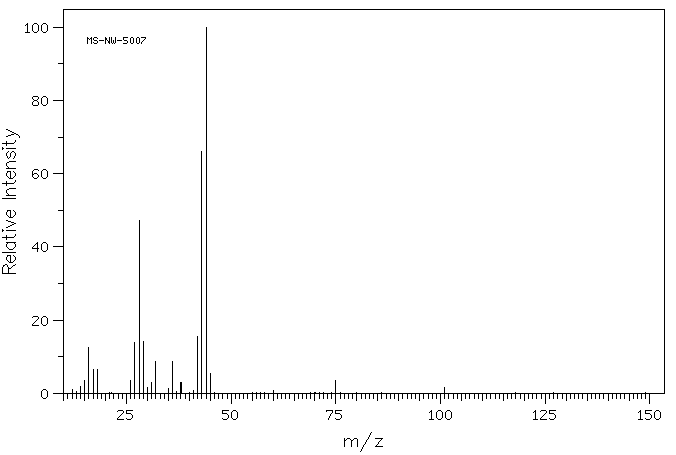
质谱MS
-
碳谱13CNMR
-
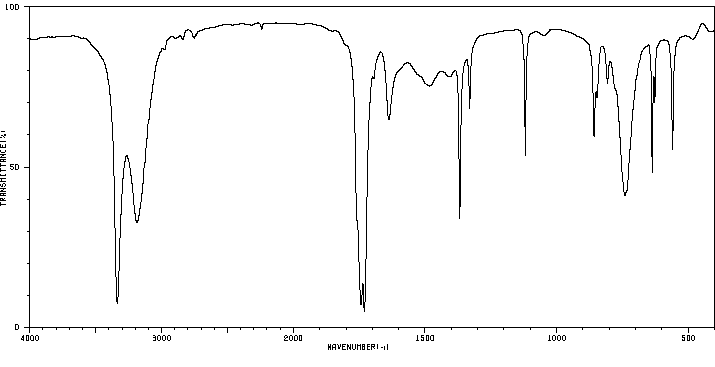
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息