2-溴-4-三氟甲基苯酚 | 81107-97-3
物质功能分类
中文名称
2-溴-4-三氟甲基苯酚
中文别名
2-溴-4-(三氟甲基)苯酚
英文名称
2-bromo-4-(trifluoromethyl)phenol
英文别名
——
CAS
81107-97-3
化学式
C7H4BrF3O
mdl
——
分子量
241.007
InChiKey
DTEDKIRYMYDIGO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:196.7±35.0 °C(Predicted)
-
密度:1.752±0.06 g/cm3(Predicted)
-
最大波长(λmax):317nm(CH3CN)(lit.)
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:12
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:4
安全信息
-
海关编码:2908199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:室温
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-Bromo-4-(trifluoromethyl)phenol
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 2-Bromo-4-(trifluoromethyl)phenol
CAS number: 81107-97-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H4BrF3O
Molecular weight: 241.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-Bromo-4-(trifluoromethyl)phenol
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H301: Toxic if swallowed
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
Avoid breathing dust/fume/gas/mist/vapours/spray
P261:
P280: Wear protective gloves/protective clothing/eye protection/face protection
P301+P310: IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P405: Store locked up
Section 3. Composition/information on ingredients.
Ingredient name: 2-Bromo-4-(trifluoromethyl)phenol
CAS number: 81107-97-3
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C7H4BrF3O
Molecular weight: 241.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对三氟甲基苯酚 4-Trifluoromethylphenol 402-45-9 C7H5F3O 162.111 3-溴-4-氟三氟甲苯 2-bromo-1-fluoro-4-(trifluoromethyl)benzene 68322-84-9 C7H3BrF4 242.998 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-溴-4-(三氟甲基)苯甲醚 2-bromo-1-methoxy-4-(trifluoromethyl)benzene 402-10-8 C8H6BrF3O 255.034 —— 2-bromo-1-ethoxy-4-(trifluoromethyl)benzene 934495-35-9 C9H8BrF3O 269.061 —— 3-bromo-5-(trifluoromethyl)benzene-1,2-diol 854472-72-3 C7H4BrF3O2 257.007 —— 2-bromo-1-phenoxy-4-(trifluoromethyl)benzene 50594-35-9 C13H8BrF3O 317.105 —— 2-bromo-1-isopropoxy-4-trifluoromethylbenzene 200956-54-3 C10H10BrF3O 283.088 —— 2-bromo-1-cyclopropylmethoxy-4-(trifluoromethyl)benzene 1268511-86-9 C11H10BrF3O 295.099 1-(苄氧基)-2-溴-4-(三氟甲基)苯 1-(benzyloxy)-2-bromo-4-(trifluoromethyl)benzene 200956-32-7 C14H10BrF3O 331.132 3-溴-2-羟基-5-(三氟甲基)苯甲醛 3-bromo-2-hydroxy-5-(trifluoromethyl)benzaldehyde 886762-43-2 C8H4BrF3O2 269.018 —— tert-butyl (2-bromo-4-trifluoromethylphenoxy)acetate 779331-60-1 C13H14BrF3O3 355.152
反应信息
-
作为反应物:描述:2-溴-4-三氟甲基苯酚 在 正丁基锂 、 ammonium acetate 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 6.0h, 生成 6-trifluoromethyl-2,2-dimethyl-4-(pyridin-2-yl)-2H-1,3-benzoxazine参考文献:名称:新型1,3-苯并恶嗪衍生物作为K +通道开放剂的合成及其生物活性。摘要:设计了一系列在C4处带有2-吡啶1-氧化物基团的1,3-苯并恶嗪衍生物新系列,以探索新型的K +通道开放剂。通过使用亚氨基三氟甲磺酸的钯(0)催化的碳-碳键形成反应与有机锌试剂进行合成,并通过新的一锅一锅的苯甲酰基吡啶的1,3-苯并恶嗪骨架形成反应进行合成。测试了这些化合物在四乙基氯化铵(TEA)中的血管舒张活性,以及BaCl2诱导的和高KCl诱导的大鼠主动脉收缩,以确定潜在的K +通道开放剂,以及在自发性高血压大鼠中的口服降压作用。1,3-苯并恶嗪核的C6处具有适当形状的吸电子基团和C7处具有甲基或卤代基团是开发最佳血管舒张剂和降血压活性所必需的。特别是,DOI:10.1248/cpb.44.734
-
作为产物:参考文献:名称:改性苯乙烯醚的钌烯烃复分解催化剂:空间和电子效应的影响摘要:合成了一系列具有修饰的异丙氧基亚苄基配体的烯烃复分解催化剂,并研究了配体对复分解速率的影响。与异丙氧基相邻的位阻增加,反应速率提高。在N-杂环卡宾配合物的情况下,在螯合氧原子和RuC键上电子密度的降低明显地促进了反应速率。含有三环己基膦配体的催化剂在亚苄基亲电性方面遵循相同的趋势,而在氧气下较高的电子密度提高了反应速率。DOI:10.1016/s0040-4020(03)01029-9
-
作为试剂:描述:溴 、 对三氟甲基苯酚 、 、 氰化铜(II) 、 、 盐酸 在 sodium hydrogensulfite 、 magnesium sulfate 、 2-溴-4-三氟甲基苯酚 、 乙酸乙酯 、 盐酸 、 silica gel 、 ethyl acetate n-hexane 作用下, 以 二氯甲烷 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 40.5h, 以to obtain 2-cyano-4-trifluoromethylphenol (34.6 g, mp. 149.5°-151° C.)的产率得到2-羟基-5-三氟甲基苯腈参考文献:名称:1,3-benzoxazine derivatives, their production and use摘要:一种新的化合物,其化学式为:##STR1## 其中 ##STR2## 是一个可选取代的苯环;R.sup.1 是一个碳环或杂环基团,通过碳-碳键、烃残基等与1,3-苯并噁嗪环的4-位相连;R.sup.2 和R.sup.3 分别是氢原子或可选取代的C.sub.1-6烷基基团等,或其盐,用于治疗和预防心脏、循环、呼吸和脑部疾病。公开号:US05270308A1
文献信息
-
Macrocyclic Modulators of the Ghrelin Receptor申请人:Ocera Therapeutics, Inc.公开号:US20180110824A1公开(公告)日:2018-04-26The present invention provides novel conformationally-defined macrocyclic compounds that have been demonstrated to be selective modulators of the ghrelin receptor (growth hormone secretagogue receptor, GHS-R1a and subtypes, isoforms and variants thereof). Methods of synthesizing the novel compounds are also described herein. These compounds are useful as agonists of the ghrelin receptor and as medicaments for treatment and prevention of a range of medical conditions including, but not limited to, metabolic and/or endocrine disorders, gastrointestinal disorders, cardiovascular disorders, obesity and obesity-associated disorders, central nervous system disorders, genetic disorders, hyperproliferative disorders and inflammatory disorders.本发明提供了一种新颖的构象定义明确的大环化合物,已经证明是生长激素分泌素受体(GHS-R1a及其亚型、异构体和变体)的选择性调节剂。本文还描述了合成这些新型化合物的方法。这些化合物可用作生长激素分泌素受体的激动剂,用于治疗和预防一系列医疗状况,包括但不限于代谢和/或内分泌紊乱、胃肠道紊乱、心血管疾病、肥胖和与肥胖相关的疾病、中枢神经系统疾病、遗传疾病、过度增殖性疾病和炎症性疾病。
-
NEW PHENYLAZETIDINECARBOXYLATE OR -CARBOXAMIDE COMPOUNDS申请人:INVENTIVA公开号:US20170066717A1公开(公告)日:2017-03-09The invention relates to compounds of formula (I). where R, R 1 , R 2 , n, A and Cy have the meanings indicated in the description. The compounds of formula (I) are Nurr-1 modulators.这项发明涉及式(I)的化合物。 其中R、R1、R2、n、A和Cy的含义如描述中所示。 式(I)的化合物是Nurr-1调节剂。
-
The discovery of a potent and selective pyrazolo-[2,3-e]-[1,2,4]-triazine cannabinoid type 2 receptor agonist作者:Michael Moir、Samuel Lane、Andrew P. Montgomery、David Hibbs、Mark Connor、Michael KassiouDOI:10.1016/j.ejmech.2020.113087日期:2021.1The development of selective CB2 receptor agonists is a promising therapeutic approach for the treatment of inflammatory diseases, without CB1 receptor mediated psychoactive side effects. Preliminary structure-activity relationship studies on pyrazoylidene benzamide agonists revealed the -ylidene benzamide moiety was crucial for functional activity at the CB2 receptor. A small library of compounds
-
[EN] ALLOSTERIC CHROMENONE INHIBITORS OF PHOSPHOINOSITIDE 3-KINASE (PI3K) FOR THE TREATMENT OF DISEASES ASSOCIATED WITH P13K MODULATION<br/>[FR] INHIBITEURS CHROMÉNONE ALLOSTÉRIQUES DE LA PHOSPHOINOSITIDE 3-KINASE (PI3K) POUR LE TRAITEMENT DE MALADIES ASSOCIÉES À LA MODULATION DE PI3K申请人:PETRA PHARMA CORP公开号:WO2021202964A1公开(公告)日:2021-10-07The disclosure relates to compounds of Formula (I) as allosteric chromenone inhibitors of phosphoinositide 3-kinase (PI3K) useful in the treatment of diseases or disorders associated with PI3K modulation, Formula (I), or a prodrug, solvate, enantiomer, stereoisomer, tautomer, or pharmaceutically acceptable salt thereof, wherein R1, R2, R3, R4, R5, R6, R7, R8, R9, W, X, Y, s, and Ring A are as described herein.
-
咪唑并环类PAR4拮抗剂及其医药用途申请人:中国药科大学公开号:CN110627817B公开(公告)日:2022-03-29本发明涉及式(I)或式(II)所示的咪唑并环类化合物、或其药学上可接受的盐或酯或溶剂化物。本发明的化合物可用于制备预防或治疗血栓栓塞性病症的药物。
表征谱图
-
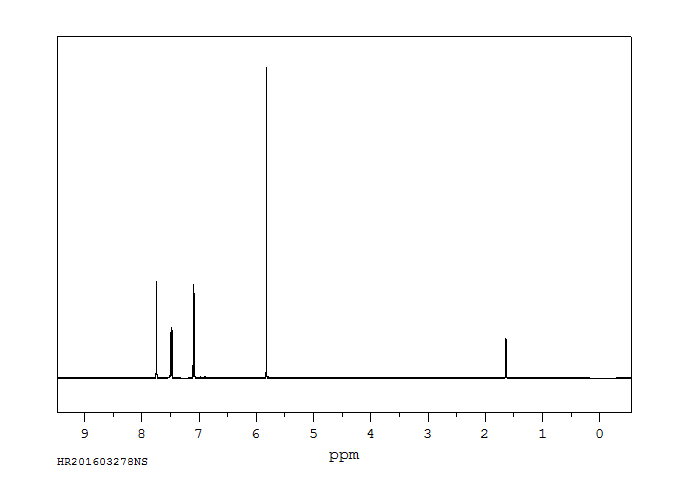
氢谱1HNMR
-
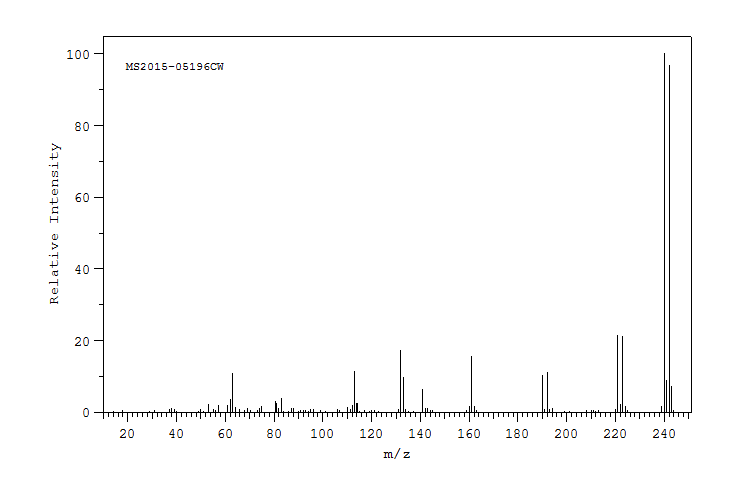
质谱MS
-
碳谱13CNMR
-
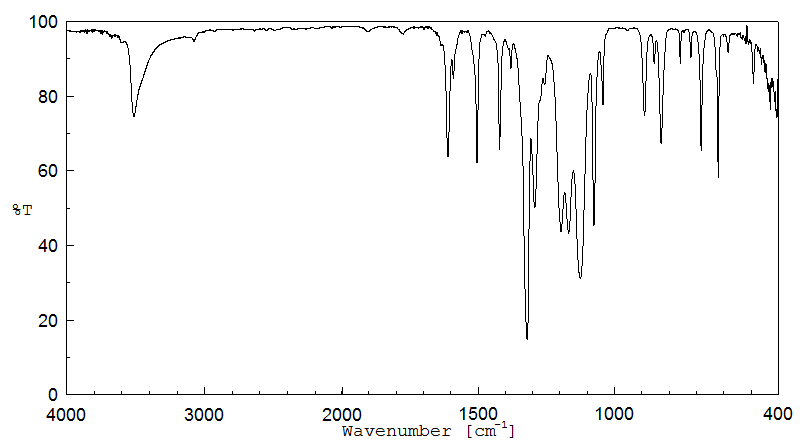
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫