1,1-二苯基-2-苦味酰肼 | 1707-75-1
中文名称
1,1-二苯基-2-苦味酰肼
中文别名
1,1-二苯基-2-苦基肼
英文名称
2,2-diphenyl-1-picrylhydrazine
英文别名
DPPH;1,1-diphenyl-2-picrylhydrazine;1,1-diphenyl-2-picrylhydrazyl;2,2-diphenyl-1-(2,4,6-trinitrophenyl)hydrazine;2,2-diphenyl-1-picrylhydrazyl;DPPH-H;1,1-diphenyl-2-(2,4,6-trinitrophenyl)hydrazine
CAS
1707-75-1
化学式
C18H13N5O6
mdl
MFCD00007231
分子量
395.331
InChiKey
WCBPJVKVIMMEQC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:~174 °C (dec.)(lit.)
-
沸点:530.2±60.0 °C(Predicted)
-
密度:1.539±0.06 g/cm3(Predicted)
-
溶解度:溶于氯仿
-
稳定性/保质期:
常温常压下稳定,避免强酸接触。
计算性质
-
辛醇/水分配系数(LogP):5.5
-
重原子数:29
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:153
-
氢给体数:1
-
氢受体数:8
安全信息
-
危险等级:4.1
-
危险品标志:Xn
-
安全说明:S36/37
-
危险类别码:R20/21/22
-
WGK Germany:3
-
海关编码:2928000090
-
储存条件:请将容器密封储存于阴凉干燥处。
SDS
1.1 产品标识符
: 1,1-二苯基-2-苦味酰肼
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
急性毒性, 吸入 (类别4)
急性毒性, 经皮 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H312 皮肤接触有害。
H332 吸入有害。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 防护服。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P312 如感觉不适,呼救中毒控制中心或医生.
P322 具体措施(见本标签上提供的急救指导)。
P330 漱口。
P363 沾染的衣服清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C18H13N5O6
分子式
: 395.33 g/mol
分子量
组分 浓度或浓度范围
N,N-Diphenyl-N'-picrylhydrazine
-
CAS 号 1707-75-1
EC-编号 216-953-2
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 174 °C - 分解
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强酸
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤被吸收是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
: 1,1-二苯基-2-苦味酰肼
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
急性毒性, 经口 (类别4)
急性毒性, 吸入 (类别4)
急性毒性, 经皮 (类别4)
2.2 GHS 标记要素,包括预防性的陈述
象形图
警示词 警告
危险申明
H302 吞咽有害。
H312 皮肤接触有害。
H332 吸入有害。
警告申明
预防
P261 避免吸入粉尘/烟/气体/烟雾/蒸气/喷雾.
P264 操作后彻底清洁皮肤。
P270 使用本产品时不要进食、饮水或吸烟。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 防护服。
措施
P301 + P312 如果吞下去了: 如感觉不适,呼救解毒中心或看医生。
P302 + P352 如与皮肤接触,用大量肥皂和水冲洗受感染部位.
P304 + P340 如吸入,将患者移至新鲜空气处并保持呼吸顺畅的姿势休息.
P312 如感觉不适,呼救中毒控制中心或医生.
P322 具体措施(见本标签上提供的急救指导)。
P330 漱口。
P363 沾染的衣服清洗后方可重新使用。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C18H13N5O6
分子式
: 395.33 g/mol
分子量
组分 浓度或浓度范围
N,N-Diphenyl-N'-picrylhydrazine
-
CAS 号 1707-75-1
EC-编号 216-953-2
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
全套防化学试剂工作服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 结晶
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 174 °C - 分解
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强酸
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入有害。 可能引起呼吸道刺激。
摄入 误吞对人体有害。
皮肤 如果通过皮肤被吸收是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
上下游信息
反应信息
-
作为反应物:描述:1,1-二苯基-2-苦味酰肼 在 盐酸 、 sodium nitrite 作用下, 以 二氯甲烷 为溶剂, 以90%的产率得到2-(p-nitrophenyl)-2-phenyl-1-(2,4,6-trinitrophenyl)hydrazine参考文献:名称:Ioniţǎ, Petre; Cǎproiu, Miron Teodor; Drǎghici, Constantin, Revue Roumaine de Chimie, 2001, vol. 46, # 7, p. 803 - 806摘要:DOI:
-
作为产物:描述:参考文献:名称:Total Antioxidant Capacity of Some Commercial Fruit Juices: Electrochemical and Spectrophotometrical Approaches摘要:本文旨在使用 DPPH-(2,2-二苯基-1-苦基肼)/DPPH(2,2-二苯基-1-苦基肼)氧化还原对偶,通过分光光度法和双计量法评估一些商用果汁(即柑橘类果汁)的总抗氧化能力。Trolox® 被选为标准抗氧化剂。分光光度法是根据 DPPH 溶液吸光度的下降进行测定。在双计量法中,研究了电位差、ΔE、DPPH- 浓度和 Trolox® 浓度等参数的影响。Trolox® 的校准图在 5 至 30 µM 之间呈线性关系(y = 0.059 x + 0.0564,其中 y 代表电流强度值,以 μA 表示;x 代表 Trolox® 浓度值,以 μM 表示;r2 = 0.9944)。生物比色法的 R.S.D. 值为 1.29%(n = 10,c = 15 μM Trolox®)。在分光光度法中,Trolox® 的校准图在 0.01 至 0.125 mM 之间呈线性关系(y = -9.5789 x+1.4533,其中 y 代表吸光度值,x 代表 Trolox® 浓度值,以 mM 表示;r2 = 0.9963)。分光光度法的 R.S.D. 值为 2.05%。这两种方法都适用于实际样品(天然果汁和软饮料)中总抗氧化活性的测定,结果非常一致。DOI:10.3390/molecules14010480
-
作为试剂:描述:参考文献:名称:Chemiluminescence in the reaction of a sulfurane with alkyl hydroperoxides摘要:DOI:10.1021/ja00530a035
文献信息
-
Methods for Producing Fluorine-Containing Hydroxyaldehyde, Fluorine-Containing Propanediol, and Fluorine-Containing Alcohol Monomer申请人:Central Glass Company, Limited公开号:US20150361026A1公开(公告)日:2015-12-17As shown by the following reaction formula, disclosed is a fluorine-containing hydroxyaldehyde production method, including the step of obtaining a fluorine-containing hydroxyaldehyde represented by the general formula (1) by reacting a fluorine-containing ketone represented by the general formula (4) and an aldehyde represented by the general formula (5) in the presence of an organic base selected from a heterocyclic compound which contains a nitrogen atom in its ring or a tertiary amine. By this production method, it is possible to obtain the fluorine-containing hydroxyaldehyde in a high yield. Furthermore, it is possible to easily obtain in high yields a fluorine-containing propanediol, which is a derivative of this fluorine-containing hydroxyaldehyde, and a fluorine-containing alcohol monomer by using the same.
-
Stopped-Flow and Spectrophotometric Study on Radical Scavenging by Tea Catechins and the Model Compounds.作者:Yasushi SENBA、Tsukasa NISHISHITA、Keiko SAITO、Hiroe YOSHIOKA、Hisashi YOSHIOKADOI:10.1248/cpb.47.1369日期:——Radical scavenging of four tea catechins, (-)-epicatechin (EC), (-)-epigallocatechin (EGC), (-)-epicatechin gallate (ECg) and (-)-eppigallocatechin gallate (EGCg), and the model compounds of their partial structure was examined against the 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical using stopped-flow and spectrophotometric methods. The number of DPPH radicals scavenged by a polyphenol molecule was larger than that of phenolic hydroxyl groups, suggesting that hydrogens which bond directly to the aromatic ring can also participate in radical scavenging. A model for the scavenging reaction was proposed in which the reaction proceeded with successive dehydrogenation from a polyphenol molecule. Analysis of the second order reaction rate constants and the activation parameters between DPPH and polyphenol at the early stage of the reaction showed that the values depended on the number of phenolic hydroxyl groups and their mutual position. Contribution of the A ring of catechins to the rate constants was estimated to be far smaller than that from the B ring. In the EGCg molecule, the B ring and the gallate group were not independent, but acted as a single group for DPPH radical scavenging.对四种茶多酚(-)-表儿茶素(EC)、(-)-表没食子儿茶素(EGC)、(-)-表儿茶素没食子酸酯(ECg) 和(-)-表没食子儿茶素没食子酸酯(EGCg)及其部分结构的模型化合物, 用停流法和分光光度法研究了它们对1,1-二苯基-2-苦基肼基(DPPH)自由基的清除作用,发现一个多酚分子清除DPPH自由基的能力比羟基的数目强,说明直接与芳香环相连的氢原子也能参与自由基清除反应。提出了一个多酚分子连续脱氢的清除反应模型。研究了早期反应阶段DPPH和多酚的二级反应速率常数和活化参数,发现其数值与酚羟基数目及其相对位置有关。儿茶素A环对速率常数的贡献比B环小得多。在EGCg分子中,B环和没食子酸基团不是独立的,而是作为一个整体对DPPH自由基起清除作用。
-
Processes for producing fluorine-containing 2,4-diols and their derivatives申请人:Komata Takeo公开号:US20050215836A1公开(公告)日:2005-09-29A process for producing a fluorine-containing 2,4-diol represented by the formula [4], wherein R 1 represents a hydrogen atom or an acyclic or cyclic alkyl group having a carbon atom number of 1 to 7; R 2 represents an acyclic or cyclic alkyl group having a carbon atom number of 1 to 7, a phenyl group, or a substituted phenyl group; and R 1 and R 2 are optionally bonded to each other to form a ring, includes reducing a hydroxy ketone represented by the formula [3], wherein R 1 and R 2 are defined as above, by hydrogen in the presence of a ruthenium catalyst.
-
Process for producing alpha-substituted acrylic norbornanyyl compounds申请人:Komata Takeo公开号:US20050131248A1公开(公告)日:2005-06-16A process for producing an α-substituted acrylic norbornanyl compound represented by the formula [3] includes reacting an α-substituted acrylic acid anhydride represented by the formula [1] with a substituted norbornanyl alcohol represented by the formula [2]. wherein R 1 represents a hydrogen atom, methyl group, ethyl group, n-propyl group, isopropyl group, n-butyl group, sec-butyl group, tert-butyl group, fluoromethyl group, difluoromethyl group, trifluoromethyl group, or perfluoroethyl group, and wherein one of R 2 , R 3 and R 4 is a CF 3 C(CF 3 )(OH)CH 2 — group, and each of the other two of R 2 , R 3 and R 4 is a hydrogen.
-
Novel Water-Soluble Diorganyl Tellurides with Thiol Peroxidase and Antioxidant Activity作者:Takahiro Kanda、Lars Engman、Ian A. Cotgreave、Garth PowisDOI:10.1021/jo990842k日期:1999.10.1at pH = 7.4 by using the coupled GSSG reductase assay. Dialkyl telluride 10 turned out to be the most efficient catalyst. Several alkyl aryl tellurides 8 were also more efficient than any of the previously tested organotellurium compounds in this model. Bulky and electron-withdrawing aryl substituents seemed to reduce activity, whereas electron-donating groups enhanced it. Alkyl aryl selenide 9 was制备了具有磺丙基的新型水溶性二芳基碲化物,烷基芳基碲化物和二烷基碲化物,发现它们具有有效的过氧化物分解和断链抗氧化能力。将双(4-羟苯基)碲化物(4)的二锂,二钠,二钾和双-四甲基铵盐在叔丁醇水溶液中用2.3当量的1,3-丙磺酸内酯处理,得到相应的双-O盐5 -磺丙基丙基二芳基碲化物。用硼氢化钠在乙醇中还原各种二芳基二碲化物。将丙磺酸内酯加到所得的硬脂酸神经酸钠中后,沉淀出相应的3-芳基碲基丙烯基丙烷磺酸钠盐8。二苯基二硒化物和二丁基二碲化物类似地反应,分别得到3-苯硒烯基丙烷磺酸(9)和4-碲辛烷磺酸(10)的钠盐。使用耦合的GSSG还原酶测定法在pH = 7.4时评估了水溶性化合物的谷胱甘肽过氧化物酶样活性。事实证明,二烷基碲化物10是最有效的催化剂。在该模型中,几种烷基芳基碲化物8也比任何先前测试过的有机碲化合物更有效。庞大的和吸电子的芳基取代基似乎降低了活性,而给电子基团则增强了活
表征谱图
-
氢谱1HNMR
-
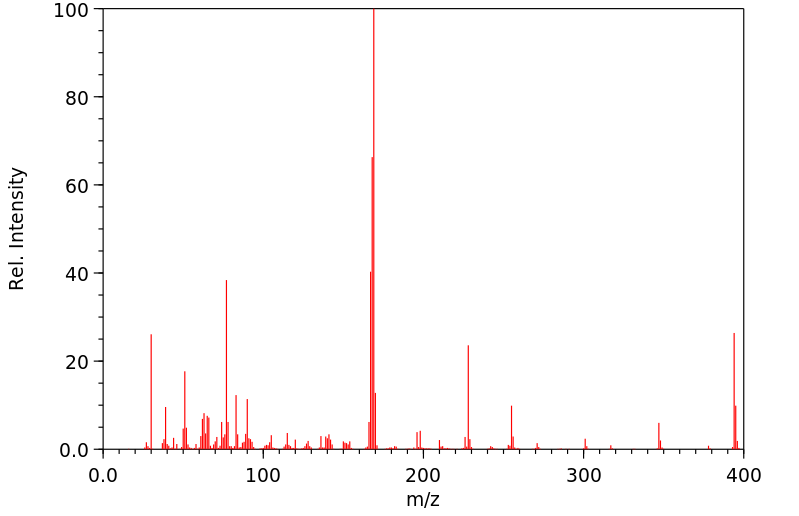
质谱MS
-
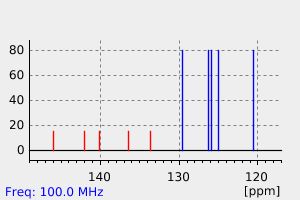
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫