4-甲基-1,3-戊二烯 | 926-56-7
中文名称
4-甲基-1,3-戊二烯
中文别名
4-甲基-1,3-戊二烯
英文名称
4-methyl-1,3-pentadiene
英文别名
4-methylpenta-1,3-diene;2-methyl-2,4-pentadiene
CAS
926-56-7;51064-12-1
化学式
C6H10
mdl
MFCD00042861
分子量
82.1454
InChiKey
CJSBUWDGPXGFGA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-94.9°C (estimate)
-
沸点:75-77 °C
-
密度:0.718 g/mL at 20 °C(lit.)
-
闪点:-18 °C
-
介电常数:2.6000000000000001
-
物理描述:Methylpentadiene appears as a clear colorless liquid with a petroleum-like odor. Less dense than water and insoluble in water. Vapors heavier than air.
-
大气OH速率常数:1.31e-10 cm3/molecule*sec
-
保留指数:609;634;636;642;636;636
计算性质
-
辛醇/水分配系数(LogP):2.7
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:3.1
-
危险品标志:Xn,F
-
危险类别码:R65,R11
-
危险品运输编号:UN 2461 3/PG 2
-
WGK Germany:3
-
海关编码:29339990
-
包装等级:II
-
危险类别:3.1
-
安全说明:S16,S36,S62
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 trans-2-甲基戊二烯 trans-2-methyl-1,3-pentadiene 926-54-5 C6H10 82.1454 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 5-methyl-hexa-1,2,4-triene 26216-05-7 C7H10 94.1564 —— (4Z)-2,7-Dimethyl-2,4,6-octatriene 38086-92-9 C10H16 136.237 —— (4E)-2,7-Dimethyl-2,4,6-octatriene —— C10H16 136.237 —— 4-Methyl-hexa-1,3-dien 4892-93-7 C7H12 96.1723 trans-2-甲基戊二烯 trans-2-methyl-1,3-pentadiene 926-54-5 C6H10 82.1454 —— (Z)-2-methyl-1,3-pentadiene 1501-60-6 C6H10 82.1454 2-甲基-2-戊烯 2-methyl-2-pentene 625-27-4 C6H12 84.1613
反应信息
-
作为反应物:描述:参考文献:名称:Alkenoyl-cyclohexadienes摘要:新的环脂烯醛酮及其在香水和香气调节剂制造中的用途,以及在一般食品制备中作为调味和调味剂,以及用于食品、饮料、动物饲料、药品制剂和烟草制品的仿味香精的制备。所述环脂烯醛酮的制备方法。公开号:US03931326A1
-
作为产物:描述:参考文献:名称:Bacon; Farmer, Journal of the Chemical Society, 1937, p. 1068,1074摘要:DOI:
-
作为试剂:描述:丙二酸环(亚)异丙酯 在 4-甲基-1,3-戊二烯 、 二甲基氯化铝 、 sodium nitrite 作用下, 以 正己烷 、 二氯甲烷 为溶剂, 反应 3.5h, 生成 Toluene-4-sulfonic acid 3,5,9,9-tetramethyl-7,11-dioxo-8,10-dioxa-1-aza-spiro[5.5]undec-3-en-1-yl ester参考文献:名称:Synthesis of Substituted Pyridines via Regiocontrolled [4 + 2] Cycloadditions of Oximinosulfonates摘要:Diels-Alder cycloadditions of oximinosulfonate 8 with a variety of 1,3-dienes proceed with regiochemistry opposite to that observed with conventional imino dienophiles, providing expeditious synthetic routes to substituted pyridines, tetrahydropyridines, and pyrrolines. The oximinosulfonate 8 is prepared in one convenient synthetic operation from Meldrum's acid and reacts with conjugated dienes at -78 degrees C in the presence of 2 equiv of dimethylaluminum chloride to afford [4 + 2] cycloadducts in good to excellent yield. Exposure of these cycloadducts to the action of NaOMe and N-chlorosuccinimide in methanol-THF at room temperature then produces substituted pyridines. The utility of this new two-step annulation protocol is demonstrated in total syntheses of the pyridine alkaloids fusaric acid and (S)-(+)-fusarinolic acid. Heating the [4 + 2] cycloadducts derived from 8 in a mixture of acetonitrile and pH 7 phosphate buffer induces an unusual Stieglitz-type rearrangement leading to the formation of interesting spirobicyclic pyrrolines.DOI:10.1021/jo981014e
文献信息
-
Enantioselective assembly of the benzo[d]xanthene tetracyclic core of anti-influenza active natural products作者:Duc Tran Ngoc、Martin Albicker、Lorenz Schneider、Nicolai CramerDOI:10.1039/c002011g日期:——A combination of an enantioselective conjugate addition/trapping sequence and a ruthenium(III)-catalyzed domino cyclization provides a concise access to benzo[d]xanthenes found in several anti-influenza active sesquiterpene natural products.
-
The diene component in the cation radical diels-alder作者:Dan W. Reynolds、Nathan L. BauldDOI:10.1016/s0040-4020(01)88079-0日期:1986.1The scope of and structural constraints upon the diene component in the cation radical Diels-Alder are investigated, with special attention to electronic, steric, and conformational effects. The major factors which control the competition between Diels-Alder and cyclobutane adduct formation are also illustrated.研究了阳离子基团Diels-Alder中二烯成分的范围和结构限制,尤其要注意电子,空间和构象效应。还说明了控制Diels-Alder与环丁烷加合物形成之间竞争的主要因素。
-
Nazarov Cyclizations of an Allenyl Vinyl Ketone with Interception of the Oxyallyl Cation Intermediate for the Formation of Carbon−Carbon Bonds作者:Vanessa M. Marx、D. Jean BurnellDOI:10.1021/ja909073r日期:2010.2.10substitution on the diene. Cyclic dienes react with the oxyallyl cation by forming only one carbon-carbon bond, but the site of bond formation can be affected by steric hindrance. Electron-rich alkenes intercept the allyl cation by forming one carbon-carbon bond, or two carbon-carbon bonds through [3 + 2] cyclization. In some instances, further treatment of the initial products with BF(3) x Et(2)O leads
-
Diastereoselective Synthesis of Seven-Membered-Ring <i>trans</i>-Alkenes from Dienes and Aldehydes by Silylene Transfer作者:Margaret A. Greene、Michel Prévost、Joshua Tolopilo、K. A. WoerpelDOI:10.1021/ja305713v日期:2012.8.1Silver-catalyzed silylene transfer to alkenes forms vinylsilacyclopropanes regioselectively. These allylic silanes undergo additions to aldehydes to form seven-membered-ring trans-alkenes with high diastereoselectivity. The high reactivity of the trans-alkenes is evidenced by their formal [1,3]-sigmatropic rearrangement reactions and the rapid additions of oxygen-hydrogen bonds across the carbon-carbon
-
An Efficient Approach to Dihydrofuroflavonoids<i>via</i>Palladium-Catalyzed Annulation of 1,3-Dienes by<i>o</i>-Iodoacetoxyflavonoids作者:Roman V. Rozhkov、Richard C. LarockDOI:10.1002/adsc.200404226日期:2004.12The palladium-catalyzed annulation of 1,3-dienes by o-iodoacetoxyflavonoids provides an efficient approach to biologically interesting dihydrofuroflavonoids. This reaction is very general, stereo- and regioselective, and a wide variety of terminal, cyclic and internal 1,3-dienes can be utilized.
表征谱图
-
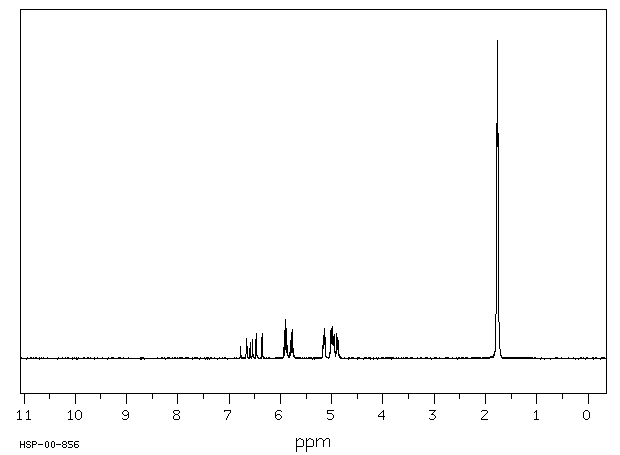
氢谱1HNMR
-
质谱MS
-
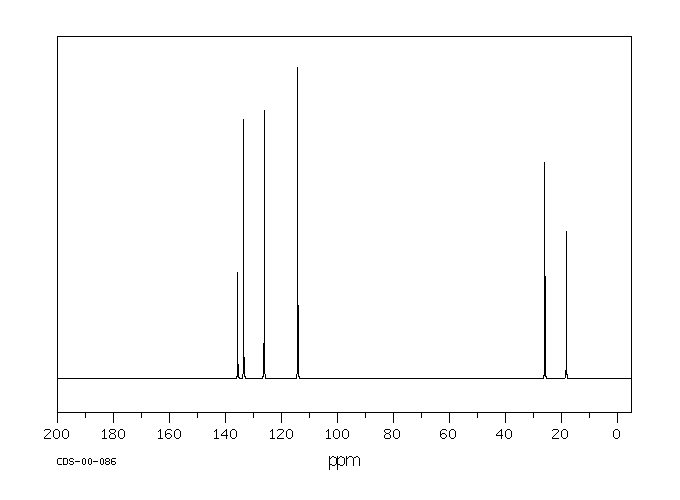
碳谱13CNMR
-
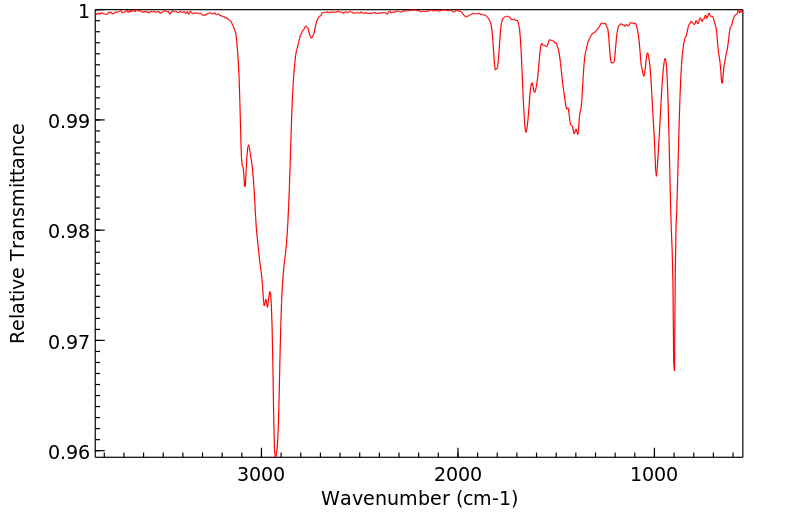
红外IR
-
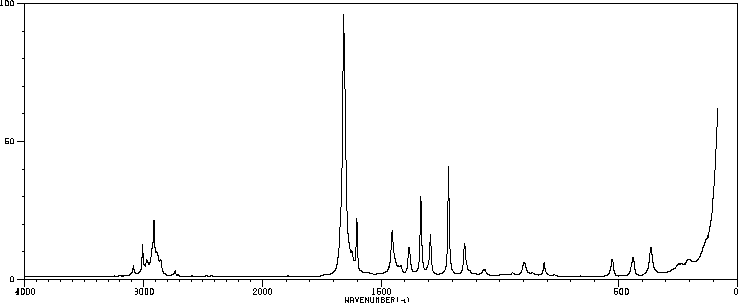
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
高密聚乙烯
香叶醇
顺式3-甲基-2-己烯
顺式-5-癸烯
顺式-5-甲基-2-己烯
顺式-5-庚烯-1-炔
顺式-4-癸烷
顺式-4-甲基-2-戊烯
顺式-4-甲基-2-戊烯
顺式-3-癸烯
顺式-3-甲基-3-己烯
顺式-3-甲基-2-庚烯
顺式-3-戊烯-1-炔
顺式-3,4-二甲基-3-己烯
顺式-3,4-二甲基-2-戊烯
顺式-3,4-二甲基-2-戊烯
顺式-2-甲基-3-己烯
顺式-2-壬烯
顺式-2-丁烯-D1
顺式-1.1.1-三甲基-2-丁烯
顺式-1-甲基-2-环丙基乙烯
顺式-1-甲基-2-乙烯基环戊烷
顺式-1-环戊基-1-辛烯
顺式-1-氘代-3-甲基-1-丁烯
顺式-(9ci)-2,3,3a,7a-四氢-4-(1-甲基乙基)-1H-茚
顺式-(2-丁烯基)环丙烷
顺式,顺式-2,4-己二烯
顺-环辛烯
顺-9-二十一碳烯
顺-6-十三碳烯
顺-5-甲基-1,3,6-庚三烯
顺-4-辛烯
顺-4-壬烯
顺-3-辛烯
顺-3-甲基-2-戊烯
顺-3-壬烯
顺-3-十三碳烯
顺-2-辛烯
顺-2-癸烯
顺-2-戊烯
顺-2-庚烯
顺-2-己烯
顺-2-丁烯
顺-2,2-二甲基-3-己烯
顺-1,3-戊二烯
顺,顺-1,9-环十六烷二烯
顺,顺,顺-环癸-1,3,5-三烯
间戊二烯
间二(4-吡啶基)苯
镁,二-2-丁烯基-