4-羟基戊烷-2-酮 | 4161-60-8
中文名称
4-羟基戊烷-2-酮
中文别名
——
英文名称
4-hydroxypentan-2-one
英文别名
4-hydroxy-2-pentanone;acetylacetone
CAS
4161-60-8
化学式
C5H10O2
mdl
——
分子量
102.133
InChiKey
PCYZZYAEGNVNMH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:2.5°C (estimate)
-
沸点:177°C
-
密度:1.0071
-
LogP:-0.385 (est)
-
保留指数:818;822;849.4
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):-0.4
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.8
-
拓扑面积:37.3
-
氢给体数:1
-
氢受体数:2
安全信息
-
海关编码:2914400090
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-羟基丁醛 acetaldol 107-89-1 C4H8O2 88.1063 2-戊酮 2-Pentanone 107-87-9 C5H10O 86.1338 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (-)-(4R)-4-羟基戊烷-2-酮 (-)-(4R)-4-hydroxypentan-2-one 63315-69-5 C5H10O2 102.133 (+)-(4S)-4-羟基戊烷-2-酮 (S)-4-hydroxypentan-2-one 73836-68-7 C5H10O2 102.133
反应信息
-
作为反应物:描述:参考文献:名称:Poray-Coschitz, Chemisches Zentralblatt, 1904, vol. 75, # I, p. 1327摘要:DOI:
-
作为产物:描述:参考文献:名称:醇274宪法(δ -甲基- Δ δ -戊烯β醇)预先解决,描述为αγγ -trimethylallyl醇摘要:DOI:10.1039/jr9380001452
文献信息
-
Silica–Dendrimer Core–Shell Microspheres with Encapsulated Ultrasmall Palladium Nanoparticles: Efficient and Easily Recyclable Heterogeneous Nanocatalysts作者:Ankush V. Biradar、Archana A. Biradar、Tewodros AsefaDOI:10.1021/la203066d日期:2011.12.6We report the synthesis, characterization, and catalytic properties of novel monodisperse SiO2@Pd-PAMAM core–shell microspheres containing SiO2 microsphere cores and PAMAM dendrimer-encapsulated Pd nanoparticle (Pd-PAMAM) shells. First, SiO2 microspheres, which were prepared by the Stöber method, were functionalized with vinyl groups by grafting their surfaces with vinyltriethoxysilane (VTS). The vinyl我们报道了新型的单分散SiO 2 @ Pd-PAMAM核-壳微球的合成,表征和催化性能,其中SiO 2 @ Pd-PAMAM核-壳微球包含SiO 2微球核和PAMAM树枝状聚合物包裹的Pd纳米粒子(Pd-PAMAM)壳。首先,将通过Stöber方法制得的SiO 2微球通过乙烯基三乙氧基硅烷(VTS)接枝其表面而被乙烯基官能化。然后将乙烯基基团通过使用转化为环氧化物米氯过氧苯甲酸。用胺封端的G4聚(酰胺基胺)(PAMAM)树状聚合物处理后,SiO 2负载的环氧化物发生开环反应,得到SiO 2@PAMAM核-壳微球。通过使Pd(II)离子与树枝状聚合物核心中的胺基络合,然后用NaBH 4将其还原为Pd(0),来合成SiO 2负载的PAMAM树状聚合物核心中的Pd纳米粒子。这产生了SiO 2 @ Pd-PAMAM核-壳微球。通过跟踪FTIR光谱,元素分析和热重曲线上的重量损失,可以监测材料中不同
-
Water-Soluble Gold-N-Heterocyclic Carbene Complexes for the Catalytic Homogeneous Acid- and Silver-Free Hydration of Hydrophilic Alkynes作者:Houssein Ibrahim、Pierre de Frémont、Pierre Braunstein、Vincent Théry、Lionel Nauton、Federico Cisnetti、Arnaud GautierDOI:10.1002/adsc.201500729日期:2015.12.14Water-soluble gold(III/I) N-heterocylic carbene complexes behave as efficient catalysts for the hydration of terminal alkynes in neat water. The transformation proceeds in the absence of Brønsted acids or halide scavengers and is suitable for sensitive substrates. Kinetic profiles and DFT studies provide a clear picture of intermediates present during catalysis.
-
A new acylation catalyst作者:Saeed Ahmad、Javed IqbalDOI:10.1039/c39870000114日期:——Cobalt(II) chloride catalyses the acylation of alcohols and amines with anhydrides in excellent yields.氯化钴(II)以优异的产率催化醇和胺与酸酐的酰化反应。
-
Tsuji–Wacker-Type Oxidation beyond Methyl Ketones: Reacting Unprotected Carbohydrate-Based Terminal Olefins through the “Uemura System” to Hemiketals and α,β-Unsaturated Diketones作者:Patrik A. Runeberg、Patrik C. EklundDOI:10.1021/acs.orglett.9b02134日期:2019.10.18Aerobic Pd(AcO)2/pyridine-catalyzed oxidation of unprotected carbohydrate-based terminal alkenes was studied. In accordance with previous reports, the initial reaction step gave methyl ketones. However, our substrates partially gave subsequent α,β-water elimination and alcohol oxidation to α,β-unsaturated 2,5-diketones. Upon increasing the pressure of O2, the reaction was shifted toward formation of
-
Oxidation of Diols and Ethers by NaBrO<sub>3</sub>/NaHSO<sub>3</sub>Reagent作者:Satoshi Sakaguchi、Daisuke Kikuchi、Yasutaka IshiiDOI:10.1246/bcsj.70.2561日期:1997.10NaBrO3 combined with NaHSO3 was found to be an excellent oxidizing reagent of alcohols, diols, and ethers under mild conditions. A variety of aliphatic and cyclic diols were selectively oxidized with satisfactory yields to the corresponding hydroxy ketones and/or diketones, which are difficult to selectively prepare due to a concomitant formation of cleaved products. For example, 2-hydroxycyclohexanone and 1,2-cyclohexanedione were selectively formed by allowing 1,2-cyclohexanediol to react with NaBrO3/NaHSO3 reagent in a selected solvent. On the other hand, an alkyl ether, such as dioctyl ether, reacted with NaBrO3/NaHSO3 in water at room temperature to give octyl octanoate in 82% yield. The same oxidation at higher temperature (60 °C) produced the α-brominated ester, octyl 2-bromooctanoate, which is considered to be formed through an alkenyl alkyl ether as the intermediate. The treatment of 1-ethoxy-1-heptene with NaBrO3/NaHSO3 afforded ethyl 2-bromoheptanoate and 2-bromoheptanoic acid as the major products.NaBrO3与NaHSO3组合被发现是一种在温和条件下对醇、二醇和醚类具有优异氧化能力的试剂。多种脂肪族和环状二醇被选择性地氧化为相应的羟基酮和/或二酮,产率令人满意,这些产物由于伴随生成断裂产物而难以选择性制备。例如,通过在选定溶剂中使1,2-环己二醇与NaBrO3/NaHSO3试剂反应,选择性地生成了2-羟基环己酮和1,2-环己二酮。另一方面,在室温下,二辛醚在水中的NaBrO3/NaHSO3反应以82%的产率得到了辛基辛酸酯。同样的氧化反应在较高温度(60°C)下产生了α-溴代酯,即辛基2-溴辛酸酯,这被认为是通过烯基烷基醚作为中间体形成的。使用NaBrO3/NaHSO3处理1-乙氧基-1-庚烯,主要产物是乙基2-溴庚酸酯和2-溴庚酸。
表征谱图
-
氢谱1HNMR
-
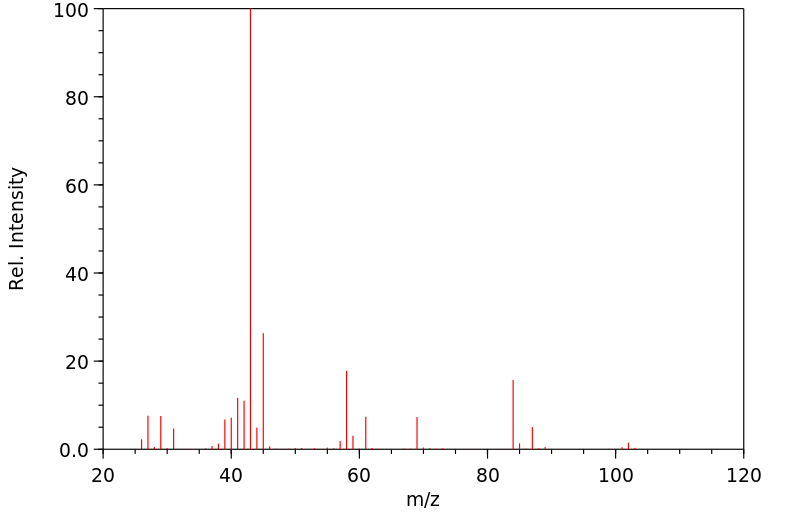
质谱MS
-
碳谱13CNMR
-
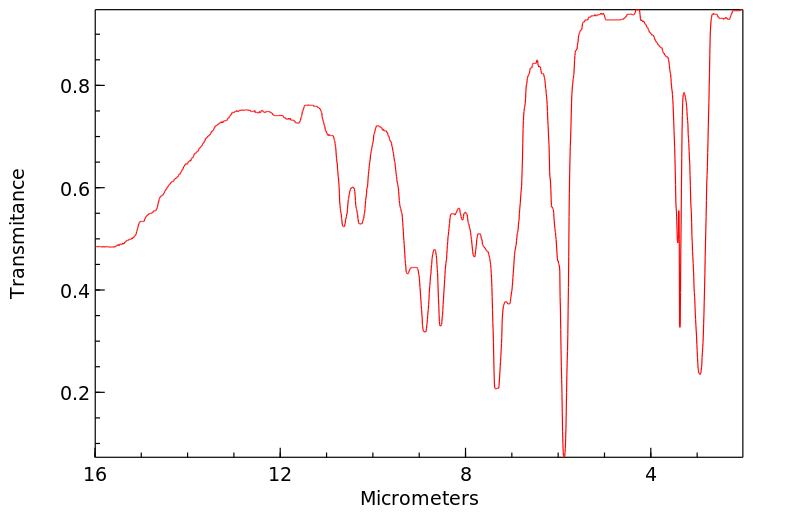
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷