2-甲基咪唑 | 693-98-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:142-143 °C (lit.)
-
沸点:267-268 °C (lit.)
-
密度:1.0500 (rough estimate)
-
闪点:155 °C
-
溶解度:780克/升
-
LogP:0.22 at 25℃
-
物理描述:DryPowder; OtherSolid
-
颜色/状态:Solid
-
蒸汽压力:6.9X10-4 mm Hg at 25 °C (est)
-
解离常数:pKa = 7.86 (conjugate acid)
-
保留指数:1050;1050
-
稳定性/保质期:
-
远离氧化物和酸。本品有毒,其毒性与二元胺相似。生产设备必须密封,防止跑、冒、滴、漏。操作人员应穿戴防护用具,避免直接接触本品。
-
本品有毒,对皮肤有致敏性反应,其毒性与二元胺类似。小鼠口服的半数致死量为1400毫克/千克,腹腔注射的半数致死量为480毫克/千克。操作人员应佩戴防护口罩和橡胶手套。
-
计算性质
-
辛醇/水分配系数(LogP):0.2
-
重原子数:6
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:28.7
-
氢给体数:1
-
氢受体数:1
ADMET
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:C
-
安全说明:S25,S26,S36/37/39,S45
-
危险类别码:R22,R34
-
WGK Germany:2
-
海关编码:2933290090
-
危险品运输编号:UN 3259 8/PG 2
-
RTECS号:NI7175000
-
包装等级:III
-
危险类别:8
-
危险标志:GHS05,GHS07,GHS08
-
危险性描述:H302,H314,H351,H360
-
危险性防范说明:P201,P280,P303 + P361 + P353,P304 + P340 + P310,P305 + P351 + P338,P308 + P313
-
储存条件:1. 存放在密封容器内,并放置在阴凉干燥处。储存地点必须上锁,钥匙应由技术专家及其助手保管。储藏时要远离氧化剂。 2. 采用铁桶或木桶包装,并内衬塑料袋,存放在阴凉通风的地方。注意防热、防晒和防潮。按照有毒化学品的规定进行贮运。
SDS
制备方法与用途
性状 2-甲基咪唑是一种白色或淡黄色的粒状或柱状晶体,相对分子质量为82.11,熔点在145~146℃之间,沸点为267℃,能升华。该物质易溶于水和醇类,微溶于冷苯,对皮肤和粘膜具有刺激性和腐蚀性。
用途 2-甲基咪唑是药物灭滴灵(甲硝哒唑)和饲料促长剂二甲唑的中间体,也是环氧树脂及其他树脂的固化剂。作为环氧树脂的中温固化剂时,可以单独使用,但主要用作粉末成型和粉末涂装的固化促进剂。
概述 2-甲基咪唑又称二甲基咪唑,在常温下为白色针状结晶或结晶性粉末。它可由乙二醛、乙醛及氨为原料制得,也可通过乙二胺和乙腈反应生成咪唑啉再脱氢而成。2-甲基咪唑是生产抗滴虫农药灭滴灵的中间体,也是环氧树脂及其他树脂的固化剂及固化促进剂。
制备方法
制法 由2-甲基咪唑啉消除脱氢而得。将2-甲基咪唑啉加热熔融(熔点107℃),小心加入活性镍,升温至200~210℃反应2小时,降温至150℃以下加水溶解,趁热压滤分离活性镍,将滤液浓缩至温度在140℃以上放料冷却即得2-甲基咪唑。用此法生产纯度为≥98%的产品,每吨产品消耗乙二胺(95%)1095kg、乙腈975kg。较好的方法是使用乙二醛和甲醛作为原料。
用途 2-甲基咪唑用作药物灭滴灵的中间体,也是环氧树脂及其他树脂的固化剂,并可作为纤维织物染料辅助剂及有机合成中的原料。
生产方法 由2-甲基咪唑啉消除脱氢而得。将2-甲基咪唑啉加热熔融(熔点107℃),小心加入活性镍,升温至200~210℃反应2小时,降温至150℃以下加水溶解,趁热压滤分离活性镍,将滤液浓缩至温度在140℃以上放料冷却即得2-甲基咪唑。用此法生产纯度为≥98%的产品,每吨产品消耗乙二胺(95%)1095kg、乙腈975kg。较好的方法是使用乙二醛和甲醛作为原料。
上下游信息
反应信息
-
作为反应物:参考文献:名称:基于咪唑核的新型高度官能化离子化合物的合成,晶体结构和抗菌活性摘要:通过咪唑,1-甲基咪唑和2-苯基-1-甲基咪唑为关键中间体,通过适当的合成途径,合成了几种新的高度官能化的咪唑鎓衍生物。用圆盘扩散法和MIC法评价了所制备化合物对大肠杆菌,金黄色葡萄球菌,铜绿假单胞菌和沙门氏菌的抗菌活性。报告了六种化合物的晶体X射线结构。DOI:10.1016/j.bmcl.2013.01.004
-
作为产物:描述:N,N'-二异丙基乙烷-1,2-二亚胺 以 乙醚 为溶剂, 800.0 ℃ 、0.04 Pa 条件下, 以43%的产率得到2-甲基咪唑参考文献:名称:的不寻常的反应性ñ -叔FVT条件下-butylimines摘要:的热反应ñ -叔丁基- (ë)-crotonaldimine(1A)和1,4-二- (叔丁基)-1,4- diazabuta -1,3-二烯(乙二醛双- ñ -叔-已经研究了FVT条件下的丁基亚胺)(1b)。已经发现,在800°C的温度下,化合物1a可能会通过均一的t -Bu –N键裂解和亚氨基自由基的形成而产生吡咯和巴豆腈。在1b在800°C的反应中,2-甲基咪唑(4b)已意外地成为主要产品。已经提出了这些反应的机理。此外,已将FVT和UV-光电子能谱结合起来用于在热活化条件下直接生成化合物1a和1b并对其进行原位表征。DOI:10.1016/j.tet.2012.10.090
-
作为试剂:描述:参考文献:名称:P-立体异构手性氧氮杂磷脂的有机催化对映选择性合成摘要:在有机催化下对映选择性合成 P-立体手性有机膦是一个具有挑战性的研究领域,使用这种方法的报道很少。在此,我们通过使用双环噻唑作为 P-N 和 P-O 键形成反应中的有机催化剂,开发了对映选择性合成 P-立体手性 oxazaphosphollides。P-手性产物以高产率制备,对映选择性适中。该过程中使用的碱对反应的对映选择性有显着影响,在某些情况下会导致 P-手性中心的相反构型。DOI:10.1002/ejoc.201600100
文献信息
-
Bioconjugates of Co(III) complexes with Schiff base ligands and cell penetrating peptides: Solid phase synthesis, characterization and antiproliferative activity作者:Dariusz Śmiłowicz、Nils Metzler-NolteDOI:10.1016/j.jinorgbio.2020.111041日期:2020.5tert.-butoxycarbonyl) in axial positions with simultaneous oxidation of Co(II) to Co(III) under ambient environment. All Co(III) complexes were characterized by multinuclear NMR spectroscopy (1H, 13C and 59Co NMR), FT-IR, mass spectrometry and HPLC. The Co(III) complexes were conjugated to three different cell penetrating peptides: FFFF (P1), RRRRRRRRRGAL (P2) and FFFFRRRRRRRRRGAL (P3). Standard solid-phase在这项工作中,我们通过水杨醛与3,4-二氨基苯甲酸(1)的单缩合反应合成了一种螯合的席夫碱。该配体在氮气下进一步用于与CoCl2·6H2O络合。在下一步中,通过使该配合物与咪唑(2),2-甲基咪唑(3)和N-Boc-1-组氨酸甲酯(4)配位(Boc:叔。 -丁氧基羰基)在轴向位置,同时在环境中将Co(II)氧化为Co(III)。所有的Co(III)配合物均通过多核NMR光谱(1H,13C和59Co NMR),FT-IR,质谱和HPLC进行表征。Co(III)复合物与三种不同的细胞穿透肽偶联:FFFF(P1),RRRRRRRRRRGAL(P2)和FFFFRRRRRRRRRGAL(P3)。标准固相肽化学用于细胞穿透肽的合成。N末端肽与钴配合物的偶联,在四齿席夫碱配体上具有羧基,提供了Co(III)-肽生物缀合物,其通过半制备HPLC纯化并通过分析HPLC和质谱法表征。与顺铂作为参考药物的活性相
-
Substituted imidazol-pyridazine derivatives申请人:——公开号:US20030229096A1公开(公告)日:2003-12-11The present invention relates to compounds of formula 1 wherein A is an unsubstituted or substituted cyclic group; and R is hydrogen or lower alkyl; or a pharmaceutically acceptable acid addition salt thereof. These compounds are NMDA NR-2B receptor subtype specific blockers and are useful in the treatment of neurodegeneration, depression and pain.本发明涉及以下式的化合物 1 其中A是未取代或取代的环状基团;以及 R是氢或较低的烷基; 或其药学上可接受的酸盐。这些化合物是NMDA NR-2B受体亚型特异性阻断剂,对于治疗神经退行性疾病、抑郁症和疼痛具有用处。
-
BRM TARGETING COMPOUNDS AND ASSOCIATED METHODS OF USE申请人:Arvinas Operations, Inc.公开号:US20190300521A1公开(公告)日:2019-10-03The present disclosure relates to bifunctional compounds, which find utility as modulators of SMARCA2 or BRM (target protein). In particular, the present disclosure is directed to bifunctional compounds, which contain on one end a ligand that binds to the Von Hippel-Lindau E3 ubiquitin ligase, and on the other end a moiety which binds the target protein, such that the target protein is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of target protein. The present disclosure exhibits a broad range of pharmacological activities associated with degradation/inhibition of target protein. Diseases or disorders that result from aggregation or accumulation of the target protein are treated or prevented with compounds and compositions of the present disclosure.本公开涉及双功能化合物,其作为SMARCA2或BRM(靶蛋白)的调节剂具有实用性。具体而言,本公开涉及包含一端结合Von Hippel-Lindau E3泛素连接酶的配体,另一端结合靶蛋白的双功能化合物,使得靶蛋白与泛素连接酶靠近以实现靶蛋白的降解(和抑制)。本公开展示了与靶蛋白降解/抑制相关的广泛药理活性。本公开的化合物和组合物用于治疗或预防由靶蛋白聚集或积累导致的疾病或紊乱。
-
Addition reaction of various azoles to perfluoromethyl vinyl ether作者:Kirill I. Petko、Andrey A. FilatovDOI:10.1007/s10593-021-02965-9日期:2021.6The addition reaction of perfluoromethyl vinyl ether to various azoles – derivatives of pyrrole, imidazole, pyrazole, indole, benzotriazole, carbazole, and triazole has been demonstrated. The reaction conditions depended both on melting point and nucleophilicity of the heterocycle. The obtained products are hydrolytically and thermally stable, have highly lipophilic moiety and can serve as useful precursors
-
[EN] N-CYCLOPROPYL-N-PIPERIDINYL-AMIDES, PHARMACEUTICAL COMPOSITIONS CONTAINING THEM AND USES THEREOF<br/>[FR] N-CYCLOPROPYL-N-PIPÉRIDINYL-AMIDES, COMPOSITIONS PHARMACEUTIQUES LES CONTENANT ET LEURS UTILISATIONS申请人:BOEHRINGER INGELHEIM INT公开号:WO2014019967A1公开(公告)日:2014-02-06The present invention relates to compounds of general formula (I), wherein R1, LP, HetAr1, (Het)Ar2 and n are as defined in the application, which have valuable pharmacological properties, and in particular bind to the GPR119 receptor and modulate its activity.本发明涉及一般式(I)的化合物,其中R1、LP、HetAr1、(Het)Ar2和n如申请中所定义,具有有价值的药理特性,特别是结合GPR119受体并调节其活性。
表征谱图
-
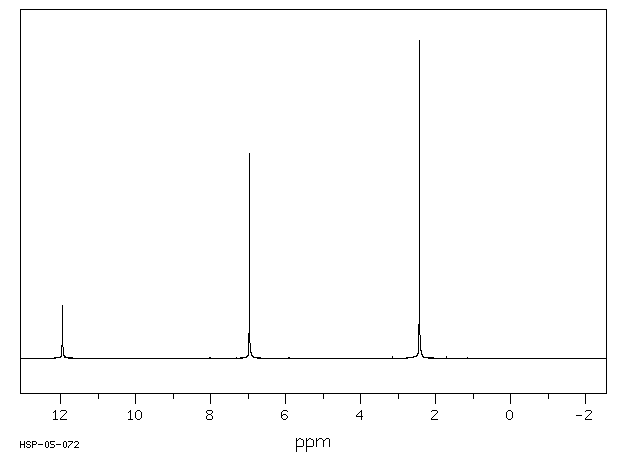
氢谱1HNMR
-
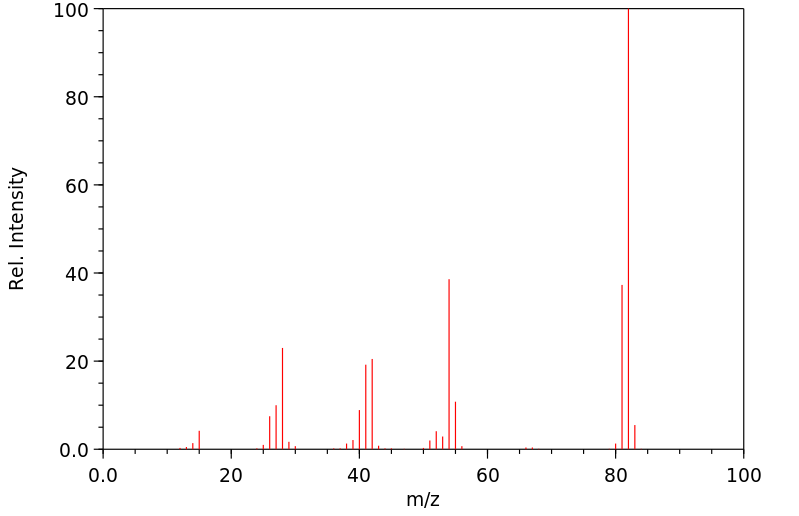
质谱MS
-
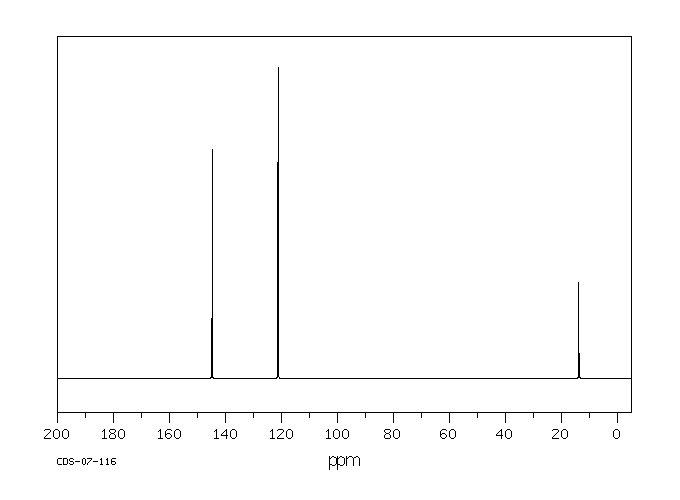
碳谱13CNMR
-
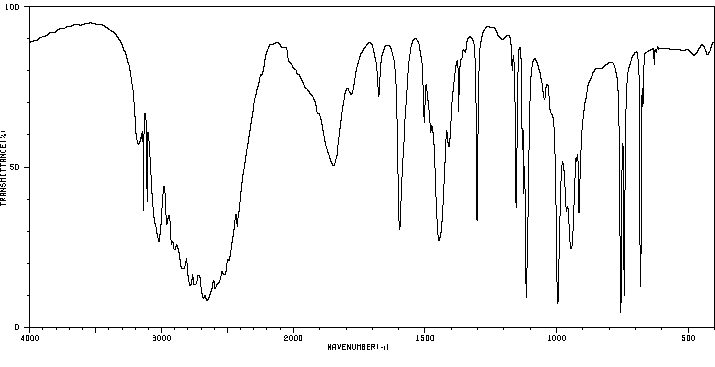
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息