lithium acetate | 546-89-4
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:283-285 °C (lit.)
-
密度:1.3[at 20℃]
-
溶解度:H2O: 5 M , 20 °C, 澄清, 无色
-
LogP:0.09 at 25℃
-
物理描述:Liquid
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):-4.24
-
重原子数:5
-
可旋转键数:0
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:40.1
-
氢给体数:0
-
氢受体数:2
ADMET
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S22,S23,S24/25,S26,S39
-
危险类别码:R36
-
WGK Germany:1
-
海关编码:29152990
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:AI5450000
-
危险性防范说明:P264,P270,P301+P312+P330,P501
-
危险性描述:H302
-
储存条件:储存于阴凉、干燥、通风良好的库房中,远离火种和热源,避免阳光直射。包装需密封,并与酸类及食用化学品分开存放,切忌混储。储区应备有合适的材料以处理泄漏物。应密闭保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 乙酸锂
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅用于研发。不作为药品、家庭或其它用途。
模块 2. 危险性概述
2.1 GHS-分类
根据全球协调系统(GHS)的规定,不是危险物质或混合物。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C2H3LiO2
分子式
: 65.99 g/mol
分子量
无
模块 4. 急救措施
4.1 必要的急救措施描述
吸入
如果吸入,请将患者移到新鲜空气处。 如呼吸停止,进行人工呼吸。
皮肤接触
用肥皂和大量的水冲洗。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者通过口喂任何东西。 用水漱口。
4.2 主要症状和影响,急性和迟发效应
高浓度的锂离子会导致头昏和虚脱,如果钠摄入有限能引起肾损伤。脱水、失重、皮肤效应和甲状腺功能紊乱
都有报道。可能出现中枢神经系统效应包括失语、视力模糊、感觉丧失、抽搐等。反复接触锂离子会发生腹泻
、呕吐和神经肌肉效应例如震颤、抽筋和反射亢进。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,抗乙醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氧化锂
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 作业人员防护措施、防护装备和应急处置程序
避免粉尘生成。 避免吸入蒸气、烟雾或气体。
6.2 环境保护措施
不要让产品进入下水道。
6.3 泄漏化学品的收容、清除方法及所使用的处置材料
扫掉和铲掉。 放入合适的封闭的容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 使容器保持密闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
常规的工业卫生操作。
个体防护设备
眼/面保护
请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟) 检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所选择身体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和数量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 283 - 285 °C - lit.
f) 沸点、初沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 蒸汽密度
无数据资料
m) 密度/相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应
无数据资料
10.4 应避免的条件
无数据资料
10.5 不相容的物质
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤刺激或腐蚀
无数据资料
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞致突变性
细胞突变性-体内试验 - 小鼠 - 经口
细胞发生分析
细胞突变性-体内试验 - 小鼠 - 经口
姐妹染色单体互换
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
实验室试验表明有畸胎生成效应
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 通过皮肤吸收可能有害。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
高浓度的锂离子会导致头昏和虚脱,如果钠摄入有限能引起肾损伤。脱水、失重、皮肤效应和甲状腺功能紊乱
都有报道。可能出现中枢神经系统效应包括失语、视力模糊、感觉丧失、抽搐等。反复接触锂离子会发生腹泻
、呕吐和神经肌肉效应例如震颤、抽筋和反射亢进。,
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: AI5450000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久性和降解性
无数据资料
12.3 潜在的生物累积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不良影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和不可回收的溶液交给有许可证的公司处理。
受污染的容器和包装
按未用产品处置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国运输名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 国际空运危规: 否
海洋污染物(是/否): 否
14.6 对使用者的特别提醒
无数据资料
参见发票或包装条的反面。
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
乙酸锂溶于水及醇,在0℃时100g水中可溶解23.76g醋酸锂。在25.8℃时,100g水中可以溶解31.28g醋酸锂;而在102.8℃时,则可溶解66.73g醋酸锂。该物质在128°C发生玻璃化转变。
制备[ \text{Li}_2\text{CO}_3 + 2 \text{CH}_3\text{COOH} → 2 \text{CH}_3\text{COOLi} + \text{H}_2\text{O} + \text{CO}_2↑ ]
水中溶解度(g/100ml)不同温度下每100毫升水中的溶解克数如下:
- 0℃:31.2g
- 10℃:35.1g
- 20℃:40.8g
- 30℃:50.6g
- 40℃:68.6g
反应信息
-
作为反应物:描述:参考文献:名称:Raj; Kapil; Bhaumik, Indian Journal of Chemistry - Section B Organic and Medicinal Chemistry, 1999, vol. 38, # 7, p. 759 - 762摘要:DOI:
-
作为产物:描述:lithium acetate dihydrate 以 solid 为溶剂, 生成 lithium acetate参考文献:名称:摘要:The thermal decompositions of a series of simple lithium compounds (carbonate, sulfate, acetate, citrates, aspartates and glutamates) currently used in the treatment of manic-depressive psychosis and related disorders were investigated by means of TG and DTA measurements in oxygen atmosphere. Pyrolysis residues were characterized by infrared spectroscopy. The stabilities of the hydrates and intermediate phases generated during the degradation processes are discussed.DOI:10.1023/a:1010192020465
-
作为试剂:描述:4-甲胺基-2-甲硫基-5-醛基嘧啶 在 哌啶 、 N-碘代丁二酰亚胺 、 lithium acetate 、 溶剂黄146 作用下, 以 乙醇 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 2.58h, 生成 6-iodo-8-methyl-2-(methylthio)pyrido[2,3-d]pyrimidin-7(8H)-one参考文献:名称:WO2024097805A1摘要:公开号:
文献信息
-
一种硫酸单烃基酯锂盐衍生物的合成方法申请人:杉杉新材料(衢州)有限公司公开号:CN112390732A公开(公告)日:2021-02-23
-
Studies of the synthesis of 5-hydroxy 6-keto steroids and related 6-keto steroids作者:Peter Yates、Shirley StiverDOI:10.1139/v87-369日期:1987.9.1
Syntheses of 5-hydroxy-5α- and 5β-cholestan-6-one (11 and 13) and their 3β-acetoxy (10 and 21) and 3β-benzyloxy derivatives (12 and 19) are described, as are syntheses of the 7α-deutero derivatives of 10 and 21. Related investigations of the syntheses of the 5-methoxy and 5-methyl analogues of these compounds are also discussed. Treatment of 12 with potassium tert-butoxide has been shown to give 5-hydroxy-5β-cholest-3-en-6-one (14) and its Δ2 isomer 15. Reaction of 6-nitrocholesteryl acetate (50) with lithium dimethylcuprate gives 3α,5-cyclo-5α-cholestan-6-one (E)-oxime (51) as the major product.
-
Epoxidation of trans-4-Aminocyclohex-2-en-1-ol Derivatives: Competition of Hydroxy-Directed and Ammonium-Directed Pathways作者:Méabh B. Brennan、Stephen G. Davies、Ai M. Fletcher、James A. Lee、Paul M. Roberts、Angela J. Russell、James E. ThomsonDOI:10.1071/ch14531日期:——directing groups on the rate of the epoxidation reaction: the diastereoisomeric ratio of the epoxide products is also the ratio of the rate constants describing the competing epoxidation processes (the group with the higher directing ability dominating the stereochemical course of the reaction). Analysis of the diastereoisomeric epoxide mixtures obtained from these reactions allowed the following order ofñ -取代反式-4-氨基环己-2-烯-1-醇的环氧化发生在用氯处理3 CCO 2小时,随后元氯过苯甲酸(米-CPBA)通过羟基和原位形成的铵离子氢键传递而产生的竞争途径。这些反应中没有环氧化物开环,使这些底物成为分析两个相互竞争的导向基团对环氧化反应速率的影响的独特平台:环氧化物产物的非对映异构体比率也是描述以下常数的比率:竞争性环氧化过程(具有较高指导能力的基团主导反应的立体化学过程)。从这些反应中获得的非对映异构体环氧混合物的分析可以定义以下顺序的导向基团能力:NHBn >> NMeBn> OH> NBn 2。由于在仲铵离子上存在两个氢键供体位点和/或为了采用最佳几何构型而增加的构象柔韧性,仲氨基和叔氨基之间的速率差异较大,与前者的优异导向能力相符。在存在烯丙基羟基的情况下,铵导向的环氧化速度比不存在烯丙基羟基的速度慢约10倍,这与存在附加的感应吸电子的杂原子可减轻烯烃的亲核性相一致。
-
Cephalosporin displacement reaction申请人:Eli Lilly and Company公开号:US04144391A1公开(公告)日:1979-03-13The present invention is directed to a process for the displacement of the acetoxy group of a cephalosporanic acid by a sulfur nucleophile, in an organic solvent and under essentially anhydrous conditions.
-
CGRP Receptor Antagonists申请人:Luo Guanglin公开号:US20090258866A1公开(公告)日:2009-10-15The disclosure generally relates to the novel compounds of formula I, including their salts, which are CGRP receptor antagonists. The disclosure also relates to pharmaceutical compositions and methods for using the compounds in the treatment of CGRP related disorders including migraine and other headaches, neurogenic vasodilation, neurogenic inflammation, thermal injury, circulatory shock, flushing associated with menopause, airway inflammatory diseases such as asthma, and chronic obstructive pulmonary disease (COPD).本公开通常涉及公式I的新化合物,包括它们的盐,这些盐是CGRP受体拮抗剂。本公开还涉及用于治疗CGRP相关疾病,包括偏头痛和其他头痛、神经源性血管扩张、神经源性炎症、热损伤、循环性休克、与更年期相关的潮红、气道炎症性疾病如哮喘和慢性阻塞性肺病(COPD)的药物组合物和方法。
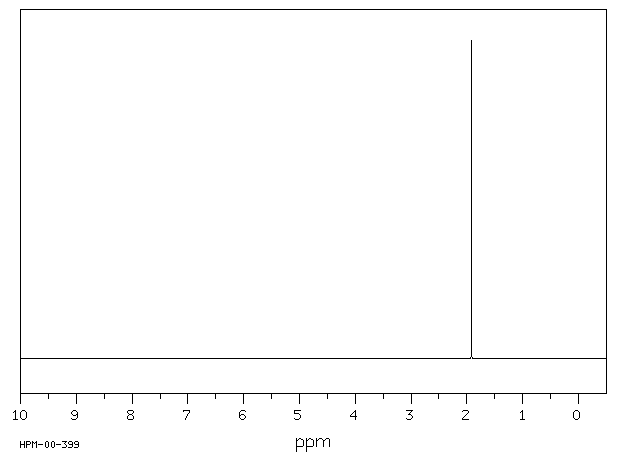
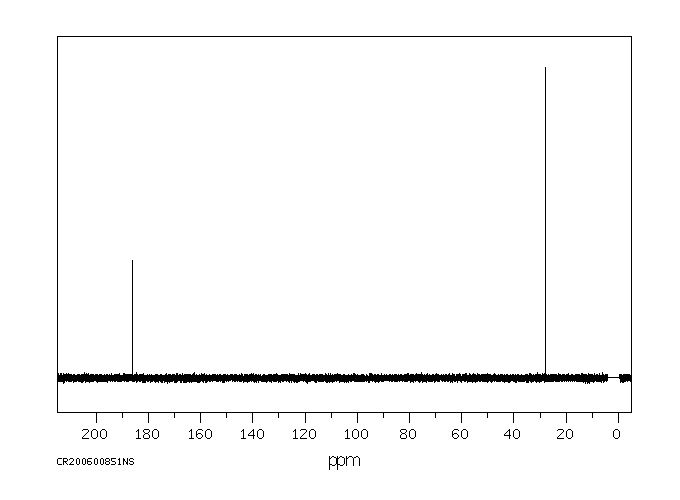
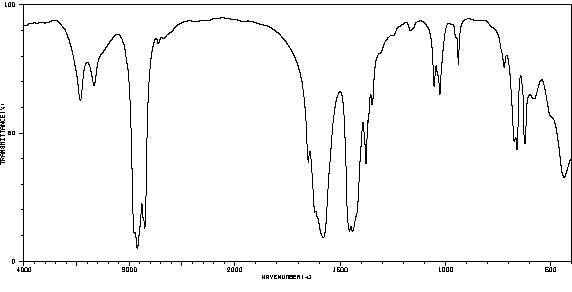
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息