potassium propionate | 327-62-8
中文名称
——
中文别名
——
英文名称
potassium propionate
英文别名
potassium propanoate;Propionsaeure-kaliumsalz;propionic acid potassium salt;potassium;propanoate
CAS
327-62-8
化学式
C3H5O2*K
mdl
——
分子量
112.17
InChiKey
BWILYWWHXDGKQA-UHFFFAOYSA-M
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:157 °C
-
密度:1.438g/cm3 at 20℃
-
溶解度:甲醇(稍微加热)、水
-
LogP:-3.23 at 25℃
-
物理描述:White crystalline powder
计算性质
-
辛醇/水分配系数(LogP):-3.85
-
重原子数:6
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:40.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
安全说明:S22,S24/25
-
危险类别码:R22
-
海关编码:2915509000
-
WGK Germany:1
-
危险性防范说明:P280
-
危险性描述:H302,H317
-
储存条件:存放于惰性气体中,并且避免接触湿气(特别是防止吸湿)。
SDS
Potassium Propionate Revision number: 5
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Potassium Propionate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Potassium Propionate
Percent: >98.0%(T)
CAS Number: 327-62-8
Synonyms: Propionic Acid Potassium Salt
Chemical Formula: C3H5KO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Potassium Propionate
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: White
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] Soluble
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Potassium Propionate
Section 10. STABILITY AND REACTIVITY
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Potassium Propionate
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: Potassium Propionate
Revision number: 5
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
Not classified
HEALTH HAZARDS
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols None
No signal word
Signal word
Hazard statements None
None
Precautionary statements:
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: Potassium Propionate
Percent: >98.0%(T)
CAS Number: 327-62-8
Synonyms: Propionic Acid Potassium Salt
Chemical Formula: C3H5KO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Potassium Propionate
Section 5. FIRE-FIGHTING MEASURES
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Handling is performed in a well ventilated place. Wear suitable protective equipment.
Technical measures:
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Keep container tightly closed. Store in a cool and dark place.
Storage conditions:
Store under inert gas.
Protect from moisture.
Store away from incompatible materials such as oxidizing agents.
Hygroscopic
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Solid
Physical state (20°C):
Form: Crystal- Powder
Colour: White
Odour: No data available
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: No data available
No data available
Flash point:
Flammability or explosive
limits:
Lower: No data available
No data available
Upper:
Relative density: No data available
Solubility(ies):
[Water] Soluble
No data available
[Other solvents]
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Potassium Propionate
Section 10. STABILITY AND REACTIVITY
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
No data available
Crustacea:
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobility in soil
No data available
Log Pow:
Soil adsorption (Koc): No data available
No data available
Henry's Law
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
Potassium Propionate
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
丙酸钾简介
丙酸钾(化学式:KC₃H₅O₂·H₂O),分子量为130.19,是一种白色潮解性晶体。其熔点为410°C,并作为丙酸的钾盐存在。该物质极易溶于水和乙醇,在120℃时会失去结晶水变成无水盐。天然存在的丙酸钾存在于发酵食品、人体汗液以及反刍动物消化物中,而工业生产则通过将丙酸溶解于氢氧化钾水溶液中来实现。
应用丙酸钾可用作食品防腐剂,在欧洲的食品标签上拥有E编码E283,在澳大利亚和新西兰则有INS编号283。它还可以作为霉菌和面包微生物性粘稠质的抑制剂,并被EEC批准用于乳品、焙烤制品及布丁等加工干酪。
鉴别试验 含量分析取经105°C干燥2小时后的样品3克(称准至mg),放入蒸馏瓶中并加入200毫升50%磷酸。煮沸2小时,并收集馏出液。通过酚酞指示剂(TS-167),使用lmol/L的NaOH溶液滴定馏出液,每1ml lmol/L NaOH液相当于丙酸钾(C₃H₅KO₂)112.17mg。
使用限量根据FAO/WHO的规定(2001年),ADI未作限制性规定。
化学性质 用途 生产方法反应信息
-
作为反应物:描述:参考文献:名称:Natta, Osterreichische Chemiker-Zeitung, vol. 40, p. 165摘要:DOI:
-
作为产物:描述:参考文献:名称:Volkova, L. D.; Vyaznikovtseva, O. V.; Gabdrakipov, V. Z., Russian Journal of Physical Chemistry, 1984, vol. 58, # 11, p. 1650 - 1652摘要:DOI:
-
作为试剂:描述:参考文献:名称:Process of condensing alcohols摘要:公开号:US01821667A1
文献信息
-
Photoinduced Electron-Transfer Reactions with Quinolinic and Trimellitic Acid Imides: Experiments and Spin Density Calculations<sup>1</sup>作者:Axel G. Griesbeck、Murthy S. Gudipati、Joachim Hirt、Johann Lex、Michael Oelgemöller、Hans Schmickler、Frank SchourenDOI:10.1021/jo001070r日期:2000.10.1products 8. The regioselectivity originates from donor-acceptor interactions prior to electron transfer and differences in spin densities in the corresponding imide radical anions. The results of DFT and ab initio calculations for the radical anions of the quinolinic acid imide (11(*)(-)) and the methyl ester of trimellitic acid imide (12(*)(-))( )()were in agreement with the latter assumption: spin densities不对称邻苯二甲酰亚胺的光诱导电子转移(PET)反应的区域选择性由中间自由基阴离子的自旋密度分布控制。发现ROHF从头算是最适合原子自旋密度分析的。用丁酸钾,己酸钾1a,b和半胱氨酸衍生物3研究了喹啉酰亚胺的分子内PET反应。形成的光环化产物2a,b和4具有适度的区域选择性(68:32、57:43和81:19 )显示优先邻环化。丙酸钾和异丁酸钾与N-甲基喹啉酰亚胺(5)的分子间反应产生二氢吡咯并[3,4-b]吡啶6a,b,其具有少量邻位区域选择性(55:45)。与这些低区域选择性相比,丙酸钾与N-甲基三苯甲基酰亚胺(9)的甲酯的PET反应仅产生对位加成产物10。同样,半胱氨酸衍生物7的分子内光反应产生了区域异构体的75:25(对位/间位)混合物环化产物8.区域选择性源自电子转移之前的供体-受体相互作用以及相应的酰亚胺自由基阴离子中自旋密度的差异。喹啉酰亚胺(11(*)(-))和偏苯三酰亚胺(
-
Cobalt-Catalyzed Acceptorless Dehydrogenation of Alcohols to Carboxylate Salts and Hydrogen作者:Deepak Ranjan Pradhan、Sandip Pattanaik、Jugal Kishore、Chidambaram GunanathanDOI:10.1021/acs.orglett.0c00193日期:2020.3.6The facile oxidation of alcohols to carboxylate salts and H2 is achieved using a simple and readily accessible cobalt pincer catalyst (NNNHtBuCoBr2). The reaction follows an acceptorless dehydrogenation pathway and displays good functional group tolerance. The amine-amide metal-ligand cooperation in cobalt catalyst is suggested to facilitate this transformation. The mechanistic studies indicate that
-
Iridium-Catalyzed Aryl C–H Sulfonamidation and Amide Formation Using a Bifunctional Nitrogen Source作者:Meng Yu、Tao Zhang、Hitesh B. Jalani、Xunqing Dong、Hongjian Lu、Guigen LiDOI:10.1021/acs.orglett.8b01977日期:2018.8.17A new strategy for the sequential formation of aryl and amidyl C–N bonds is reported. Using trichloroethoxysulfonyl azide as a bifunctional nitrogen source, Ir-catalyzed aryl C–H sulfonamidation and subsequent desulfonative amide formation proceed effectively without any need of oxidants or coupling reagents. This protocol is suitable for readily available benzamides and stable carboxylates including
-
[EN] FLUORINE-CONTAINING ESTERS AND METHODS OF PREPARATION THEREOF<br/>[FR] ESTERS FLUORÉS, ET PROCÉDÉS D'ÉLABORATION DE CEUX-CI申请人:DU PONT公开号:WO2013180782A1公开(公告)日:2013-12-05A method for preparing fluorine-containing carboxylic acid esters is described in which a salt of a carboxylic acid is reacted with a fluorinated alkyl halide. The fluorine-containing carboxylic acid esters prepared by the method disclosed herein are particularly useful as electrolyte solvents for electrochemical cells, such as a lithium ion battery, where a high purity solvent is desired.
-
One-Pot Synthesis of Phenacyl Esters from Acetophenone, [Bmim]Br<sub>3</sub>, and Potassium Salts of Carboxylic Acids Under Solvent-Free Conditions作者:Zhang-Gao Le、Zong-Bo Xie、Jian-Ping XuDOI:10.1080/00397910802431115日期:2009.1.28Abstract One-pot synthesis of phenacyl esters from acetophenone, [Bmim]Br3, and potassium salts of carboxylic acids under solvent-free conditions gave the corresponding phenacyl esters with excellent yields.
表征谱图
-
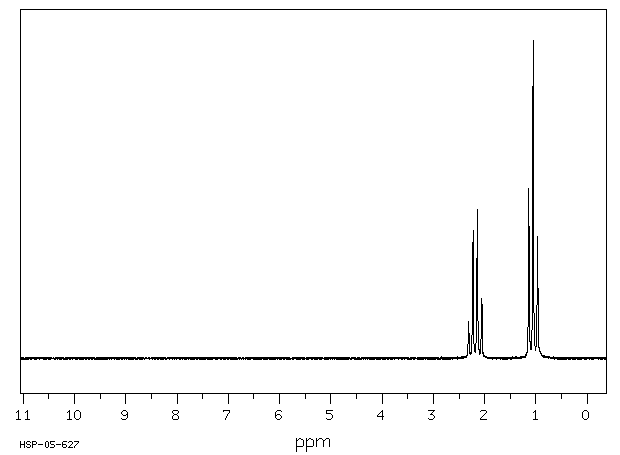
氢谱1HNMR
-
质谱MS
-
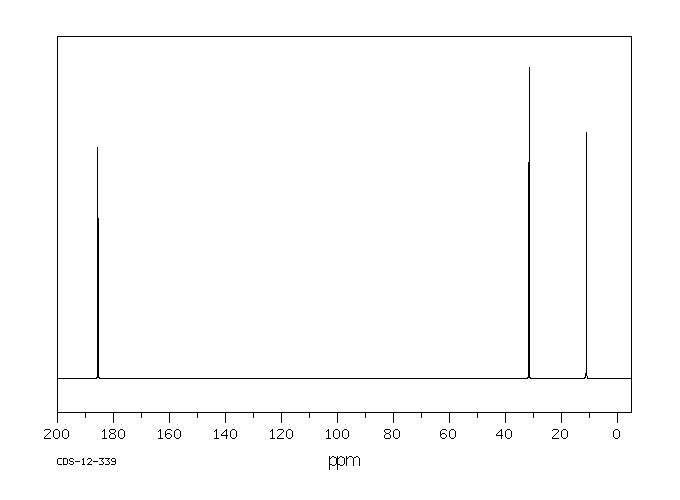
碳谱13CNMR
-
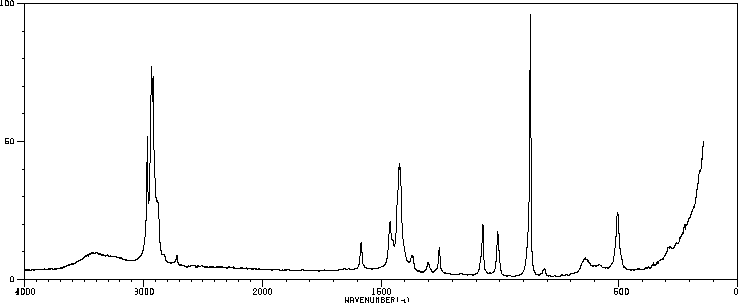
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸