L-亮氨酰甘氨酰甘氨酸 | 1187-50-4
中文名称
L-亮氨酰甘氨酰甘氨酸
中文别名
L-白氨酰甘氨酰甘氨酸
英文名称
L-Leu-Gly-Gly
英文别名
Leu-Gly-Gly;2-[[2-[[(2S)-2-azaniumyl-4-methylpentanoyl]amino]acetyl]amino]acetate
CAS
1187-50-4
化学式
C10H19N3O4
mdl
MFCD00065942
分子量
245.279
InChiKey
VWHGTYCRDRBSFI-ZETCQYMHSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:~220 °C (dec.)
-
沸点:573.7±45.0 °C(Predicted)
-
密度:1.199±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-3
-
重原子数:17
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.7
-
拓扑面积:122
-
氢给体数:4
-
氢受体数:5
安全信息
-
WGK Germany:3
-
海关编码:2924199090
SDS
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Low-molecular-weight proteins as carriers for renal drug targeting. Preparation of drug-protein conjugates and drug-spacer derivatives and their catabolism in renal cortex homogenates and lysosomal lysates摘要:Low molecular weight proteins (LMWPs) are known to be reabsorbed and catabolized primarily by the proximal tubular cells of the kidneys. As such, LMWPs might serve as drug carriers that release drugs site-specifically in the kidney. We tested this concept in vitro by coupling different drugs to the LMWP lysozyme both directly (amide bond) and via different spacers: oligopeptides (amide bond), (poly-)alpha-hydroxy acids (ester bond), and a pH sensitive cis-aconityl spacer (amide bond). The capability of the kidney to release the parent drug from such drug-spacer derivatives and drug-LMWP conjugates by enzymatic or chemical hydrolysis of the bond was tested by incubation experiments in renal cortex homogenates and lysosomal lysates. Directly coupled conjugates of terminal carboxyl group containing drugs and lysozyme were catabolized to single amino acids, but did not result in release of the parent drug. The amide bond between the drug and the final amino acid (lysine) appeared to be stable in the incubation milieu. Different oligopeptide spacers coupled to the drugs showed similar results: the oligopeptide itself was cleaved but the amide bond between the drug and different single amino acids remained untouched. Only amide bonds of derivatives of carboxylic drugs with peptide structures themselves were cleaved. Some of the directly coupled conjugates of terminal amino drugs and oligopeptides showed clear release of the parent drug whereas others were stable. Terminal amino drugs were rapidly released from an acid-sensitive cis-aconityl spacer. Terminal carboxyl group containing drugs were enzymatically released from their glycolic and lactic ester spacers at different rates. These kinds of drugs were also released as parent drug from LMWP conjugates with ester spacers like L-lactic acid. Increasing spacer length by intercalating a tetra(L-lactic acid) molecule between the drug and the protein further increased the extent and rate of drug release, indicating increased accessability of the bond to the enzymes. Terminal amino group containing drugs were rapidly generated as parent drug from LMWP conjugates using an acid-sensitive spacer. In addition the conjugates were found to be adequately stable in plasma, considering their rapid clearance from the bloodstream. It is concluded that LMWPs may indeed be of use as carriers for specific renal delivery of drugs, since renal cortex homogenates and lysosomal lysates are able to catabolize the protein and generate the parent drug from drug-LMWP conjugates bearing suitable spacers. The option of enzymatic release is limited by the narrow specificity of the lysosomal enzymes. This has profound implications for the synthesis of suitable conjugates, since the nature of the drug itself, the type of bond, and also spacer length largely determine whether release of the parent drug will occur. Tailor-made spacers containing the correct mode of attachment and the right spacer length are required for this option. Chemical hydrolysis, using acid-sensitive linkers, is suggested as a viable alternative approach.DOI:10.1021/jm00085a012
-
作为产物:描述:参考文献:名称:Grassmann; Wuensch, Chemische Berichte, 1958, vol. 91, p. 449,453摘要:DOI:
文献信息
-
Inhibition of iodine-125 labeled ristocetin binding to Micrococcus luteus cells by the peptides related to bacterial cell wall mucopeptide precursors: quantitative structure-activity relationships作者:Ki-Hwan Kim、Yvonne Martin、Ellen Otis、James MaoDOI:10.1021/jm00121a018日期:1989.1Quantitative structure-activity relationships (QSAR) of N-Ac amino acids, N-Ac dipeptides, and N-Ac tripeptides in inhibition of 125I-labeled ristocetin binding to Micrococcus luteus cell wall have been developed to probe the details of the binding between ristocetin and N-acetylated peptides. The correlation equations indicate that (1) the binding is stronger for peptides in which the side chain of
-
Linear energy correlations and failures in the low-energy tandem mass spectra of protonatedN-benzoylated tripeptides: Tools for probing mechanisms of CAD processes作者:Daniel G. Morgan、Maurice M. BurseyDOI:10.1002/jms.1190300410日期:1995.4The backbone cleavages for three series of protonated N-benzoyl tripeptide ions were studied in a hybrid tandem mass spectrometer: (i) benzoyl-Gly-Gly-Xxx, where Xxx = Gly, Ala, Val, Leu, Ile, Phe, Tyr, Met, Glu, Pro and Trp, (ii) benzoyl-Gly-Xxx-Gly, where Xxx = Gly, Ala, Leu, Phe, Tyr, Met and Trp, and (iii) benzoyl-Xxx-Gly-Gly, where Xxx = Gly, Ala, Val, Leu, Ile, Phe, Tyr, Met, Pro and Trp. C-Terminal y-type ions and N-terminal a- and b-type ions were noted in all three cases. For benzoyl-Gly-Gly-Xxx, a linear relationship between log (y1/b2) and the proton affinity of the C-terminal amino acid substituents was found: as the proton affinity of the C-terminal residue increases, the fraction of y1 ion formation increases. A similar relationship was noted for the benzoyl-Xxx-Gly-Gly tripeptides between log (y2/b1) and the proton affinity of the N-terminal amino acid substituent: as the proton affinity of the N-terminal residue increases, the fraction of b1 ion formation increases. For the series benzoyl-Gly-Xxx-Gly, these relationships did not hold true. These observations point to similar reaction pathways throughout the benzoyl-Gly-Gly-Xxx series and also similar pathways throughout the benzoyl-Xxx-Gly-Gly, but pathways that are substituent dependent for benzoyl-Gly-Xxx-Gly. The increased correlation coefficients for benzoyl-Gly-Gly-Xxx and benzoyl-Xxx-Gly-Gly when compared with the free tripeptides, suggest that fewer interfering competitive reactions exist, as fewer possibilities for internal hydrogen bonding exist in the N-benzoyl derivatives versus the free compounds.在一台混合串联质谱仪上,研究了三种系列的质子化N-苯甲酰三肽离子的骨架断裂:(i)苯甲酰-Gly-Gly-Xxx,其中Xxx = Gly、Ala、Val、Leu、Ile、Phe、Tyr、Met、Glu、Pro和Trp,(ii)苯甲酰-Gly-Xxx-Gly,其中Xxx = Gly、Ala、Leu、Phe、Tyr、Met和Trp,以及(iii)苯甲酰-Xxx-Gly-Gly,其中Xxx = Gly、Ala、Val、Leu、Ile、Phe、Tyr、Met、Pro和Trp。在所有三种情况下,都观察到了C端y型离子和N端a型及b型离子。对于苯甲酰-Gly-Gly-Xxx,发现log(y1/b2)与C端氨基酸取代基的质子亲和力之间存在线性关系:随着C端残基的质子亲和力增加,y1离子的形成分数增加。对于苯甲酰-Xxx-Gly-Gly三肽,log(y2/b1)与N端氨基酸取代基的质子亲和力之间存在类似的关系:随着N端残基的质子亲和力增加,b1离子的形成分数增加。对于苯甲酰-Gly-Xxx-Gly系列,这些关系并不成立。这些观察结果表明,在苯甲酰-Gly-Gly-Xxx系列和苯甲酰-Xxx-Gly-Gly系列中存在类似的反应途径,但对于苯甲酰-Gly-Xxx-Gly,途径依赖于取代基。与自由三肽相比,苯甲酰-Gly-Gly-Xxx和苯甲酰-Xxx-Gly-Gly的相关系数增加,表明存在的干扰竞争反应较少,因为在N-苯甲酰衍生物中内部氢键的可能性比自由化合物少。
-
[EN] N-ALKYLATED AMINO ACIDS AND OLIGOPEPTIDES, USES THEREOF AND METHODS FOR PROVIDING THEM.<br/>[FR] ACIDES AMINÉS N-ALKYLÉS ET OLIGOPEPTIDES, LEURS UTILISATIONS ET LEURS PROCÉDÉS DE PRODUCTION申请人:UNIV GRONINGEN公开号:WO2018178397A1公开(公告)日:2018-10-04The invention relates to the synthesis of amphiphilic amino acid derivatives, in particular to a method for the N-alkylation of an unprotected amino acid or the N-terminus of an oligopeptide substrate, comprising reacting said unprotected amino acid or oligopeptide substrate with an alcohol, e.g. a fatty alcohol, in the presence of a homogeneous transition metal catalyst.
-
Potential<scp>d,l</scp>-Amino Acid Sequence Analysis of Peptides from the C-Terminus作者:Hiroshi Ohrui、Eigo Itoh、Yoshihiro Nishida、Hiroko Horie、Hiroshi MeguroDOI:10.1271/bbb.61.392日期:1997.1A model tripeptide, Gly-L-Leu-L-Phe, was immobilized with activated aminomethyl polystyrene, and its C-terminal was reduced to an alcohol. This peptidyl alcohol was selectively hydrolyzed at the C-terminal amide bond to afford a polymer-supported dipeptide (Gly-L-Leu) and amino alcohol (Phe-OH). The amino alcohol, including its absolute configuration, was determined by labelling with (+)-MNB-COOH, and the dipeptide was reused for a determination of its C-terminal amino acids. The d, l-amino acids of the tripeptide were sequentially determined from the C-terminus.
-
Synthesis of tripeptide derivatized cyclopentadienyl complexes of technetium and rhenium as radiopharmaceutical probes作者:Qaisar Nadeem、Daniel Can、Yunjun Shen、Michael Felber、Zaid Mahmood、Roger AlbertoDOI:10.1039/c3ob41866a日期:——We describe the syntheses of half-sandwich complexes of the type [(η5-Cp(CONH-R))M(CO)3] with M = Re or 99mTc. The R group represents different tri-peptides (tpe) which display high binding affinities for oligopeptide transporters PEPT2. The 99mTc complexes were prepared directly from [99mTc(OH2)3(CO)3]+ and Diels–Alder dimerized, cyclopentadienyl derivatized peptides in water. This approach corroborates the feasibility of metal-mediated retro Diels–Alder reactions for the preparation of not only small molecules but also peptides carrying a [(η5-Cp)99mTc(CO)3] tag. We synthesized the Diels–Alder product [(HCpCONH-tpe)2] from Thiele's acid [(η5-HCpCOOH)2] via double peptide coupling. The Re-complexes [(η5-CpCONH-tpe)Re(CO)3] were obtained by attaching [(Cp-COOH)Re(CO)3] directly to the N-terminus of peptides as received from SPPS. The authenticity of the 99mTc-complexes is confirmed by chromatographic comparison with the corresponding rhenium complexes, fully characterized by spectroscopic techniques.我们描述了[(η5-Cp(CONH-R))M(CO)3]型半三明治复合物的合成,其中M = Re或99mTc。R基团代表不同的三肽(tpe),它们对寡肽转运蛋白PEPT2具有高结合亲和力。99mTc复合物直接由[99mTc(OH2)3(CO)3]+和Diels-Alder二聚体、环戊二烯衍生物肽在水中制备。这种方法证实了金属介导的反式Diels-Alder反应不仅可用于制备小分子,还可用于制备带有[(η5-Cp)99mTc(CO)3]标签的肽。我们通过双肽偶联从Thiele酸[(η5-HCpCOOH)2]合成了Diels-Alder产物[(HCpCONH-tpe)2]。通过将[(Cp-COOH)Re(CO)3]直接连接到从SPPS获得的肽的N端,获得了Re复合物[(η5-CpCONH-tpe)Re(CO)3]。通过与相应的铼复合物进行色谱比较,证实了99mTc复合物的真实性,并通过光谱技术对其进行了充分表征。
表征谱图
-
氢谱1HNMR
-
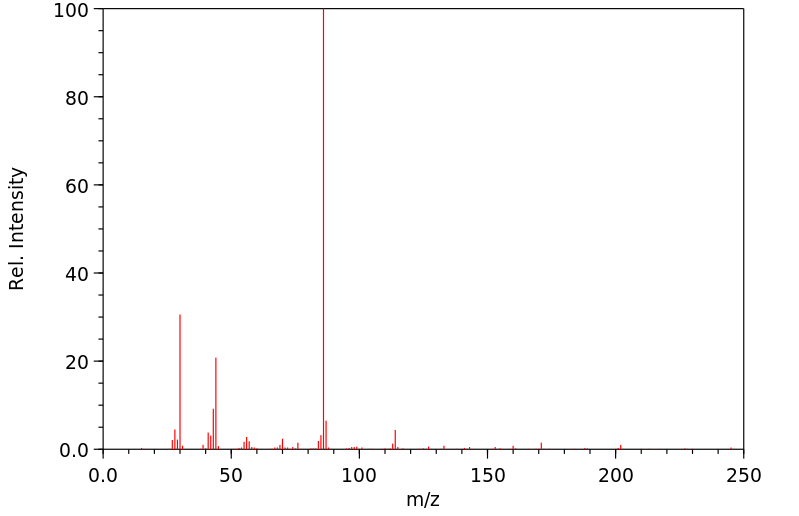
质谱MS
-
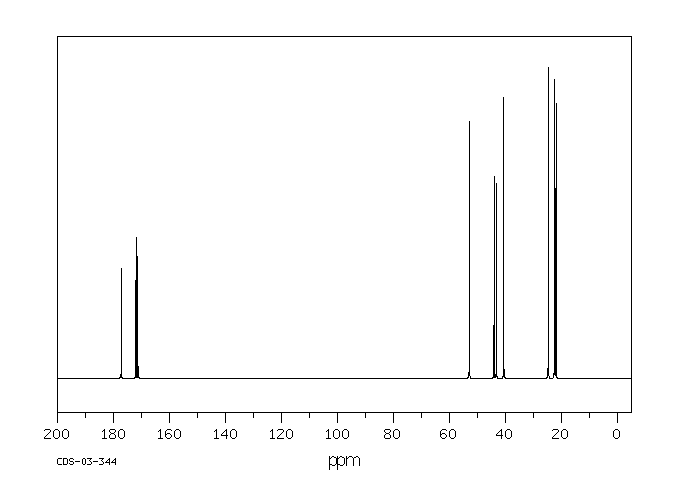
碳谱13CNMR
-
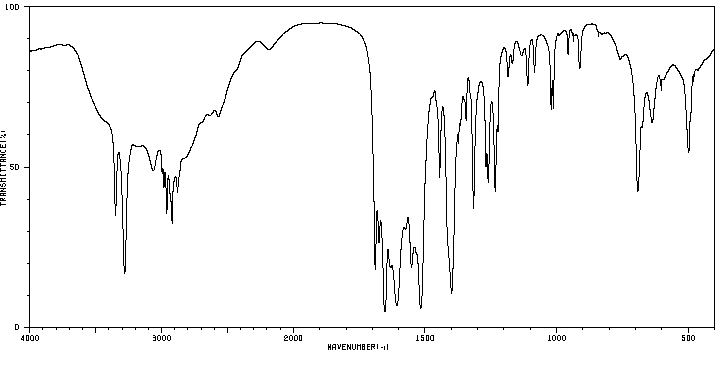
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸