2,3-戊二酮 | 600-14-6
中文名称
2,3-戊二酮
中文别名
2,3-戊烷二酮;乙酰丙酰;乙酰基丙酰
英文名称
2,3-Pentanedione
英文别名
pentane-2,3-dione;pentan-2,3-dione;ethyl methyl diketone
CAS
600-14-6
化学式
C5H8O2
mdl
——
分子量
100.117
InChiKey
TZMFJUDUGYTVRY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-52 °C (lit.)
-
沸点:110-112 °C (lit.)
-
密度:0.957 g/mL at 25 °C (lit.)
-
闪点:66 °F
-
溶解度:水中的溶解度为60克/升
-
暴露限值:NIOSH: TWA 9.3 ppb; STEL 31 ppb
-
LogP:-0.85
-
物理描述:Liquid
-
颜色/状态:Yellow liquid
-
气味:Somewhat sweet odor similar to quinone ... Detection: 20 ppb. Aroma characteristics at 1.0%: buttery diacytl-like, fermented dairy and creamy, popcorn buttery
-
味道:Penetrating buttery taste on dilution...Taste characteristics at 1 to 5 ppm: sweet buttery, creamy, cheesy, slightly toasted dairy, with a rich baked goods nuance and good mouth feel
-
蒸汽压力:2.67 kPa at 20 °C /20.0 mm Hg at 20 °C/
-
稳定性/保质期:
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
折光率:Index of refraction: 1.4014 at 19 °C
-
保留指数:660;666;669;681;665;662;664;650;668;662;674;675;667;660;675;665;665;681;668.7;678.1;680;668;672;664;674;662;664;673;674;651;652;673;674;681;663;681;673;676;681;678.1;675.8
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
ADMET
代谢
我们开发了一个系统,用于将培养的人体支气管/气管上皮细胞(NHBEs)暴露于调味蒸气中。NHBEs暴露于二乙酰或2,3-戊二酮蒸气中6小时(25或大于/等于60 ppm)...对基底外侧介质的分析表明,NHBEs将二乙酰和2,3-戊二酮分别代谢为乙偶姻和2-羟基-3-戊酮。
We developed a system to expose cultured human bronchial/tracheal epithelial cells (NHBEs) to flavoring vapors. NHBEs were exposed for 6 hr to diacetyl or 2,3-pentanedione vapors (25 or >/= 60 ppm)... Analysis of the basolateral medium indicated that NHBEs metabolize diacetyl and 2,3-pentanedione to acetoin and 2-hydroxy-3-pentanone, respectively.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
识别和使用:2,3-戊二酮是一种黄色液体。它被用作调味剂(黄油风味),包括许多电子烟品牌。人类暴露和毒性:微波爆米花行业的工人吸入黄油风味可能导致“爆米花工人肺”。将培养的人支气管/气管上皮细胞(NHBEs)暴露于二乙酰或2,3-戊二酮蒸气(25或≥60 ppm)6小时,并测量对短路电流和跨上皮电阻(Rt)的影响。暴露于25 ppm后,两种风味剂立即减少了Na+的传输,而不影响Cl-的传输或Na+,K+-泵的活动。据报道,二乙酰和2,3-戊二酮的浓度(100-360 ppm)在体内引起上皮损伤,而60 ppm导致NHBEs在暴露后0小时死亡。结果表明,与体内或体外细胞形态变化的浓度相比,较低浓度的风味剂暂时抑制了气道上皮细胞的离子传输。动物研究:大鼠吸入空气,2,3-戊二酮(112、241、318或354 ppm)6小时后次日处死。吸入2,3-戊二酮的大鼠发展为坏死性鼻炎、气管炎和支气管炎。为了研究延迟毒性,另外一组大鼠吸入318(范围317.9-318.9)ppm 2,3-戊二酮6小时,在暴露后0至2、12至14或18至20小时处死。上鼻呼吸上皮损伤涉及凋亡和坏死,在暴露后12至14小时进展。嗅觉神经上皮损伤包括与支撑细胞相比,表达2,3-戊二酮代谢酶的二碳/L-木糖还原酶减少的嗅觉神经元的丢失。半胱天冬酶3激活偶尔涉及在嗅球(OB)突触的嗅觉神经束。另一组大鼠吸入270 ppm 2,3-戊二酮6小时41分钟后,使用实时PCR观察到嗅球、纹状体、海马和脑桥中的IL-6和一氧化氮合酶-2表达增加,血管内皮生长因子A表达减少。紧密蛋白-1在嗅球和纹状体的表达增加。在另一实验中,雄性和雌性大鼠和小鼠每天暴露于0、50、100或200 ppm 2,3-戊二酮6小时,每周5天,最多2周。在1、3、5和10次暴露后收集支气管肺泡灌洗液(BALF),在12次暴露后评估组织病理学。在5天和10天暴露于200 ppm的大鼠BALF中,MCP-1、MCP-3、CRP、FGF-9、纤维蛋白原和OSM增加了2至9倍。在小鼠中,仅在暴露于200 ppm 5天后纤维蛋白原增加。在所有暴露于200 ppm的大鼠和小鼠中,呼吸道上皮是毒性的部位。值得注意的是,2,3-戊二酮还引起了大鼠的腔内和壁内纤维化气道病变。在第三个实验中,暴露于150或200 ppm 2,3-戊二酮的大鼠发展为支气管纤维化。在小鼠中,当测试浓度高达50%时,2,3-戊二酮不是皮肤刺激物。然而,在暴露于2,3-戊二酮后,观察到小鼠淋巴细胞的浓度依赖性增加。
IDENTIFICATION AND USE: 2,3-Pentanedione is a yellow liquid. It is used as flavoring agent (butter flavoring) including many e-cigarette brands. HUMAN EXPOSURE AND TOXICITY: Inhalation of butter flavoring by workers in the microwave popcorn industry may result in "popcorn workers' lung." Cultured human bronchial/tracheal epithelial cells (NHBEs) were exposed for 6 hours to diacetyl or 2,3-pentanedione vapors (25 or >/= 60 ppm) and the effects on short circuit current and transepithelial resistance (Rt) were measured. Immediately after exposure to 25 ppm both flavorings reduced Na+ transport, without affecting Cl- transport or Na+,K+-pump activity. Concentrations (100-360 ppm) of diacetyl and 2,3-pentanedione reported to give rise in vivo to epithelial damage, and 60 ppm, caused death of NHBEs 0 hours post-exposure. The results indicate that ion transport is inhibited transiently in airway epithelial cells by lower concentrations of the flavorings than those that result in morphological changes of the cells in vivo or in vitro. ANIMAL STUDIES: Rats that inhaled air, 2,3-pentanedione (112, 241, 318, or 354 ppm) for 6 hours were sacrificed the following day. Rats inhaling 2,3-pentanedione developed necrotizing rhinitis, tracheitis, and bronchitis. To investigate delayed toxicity, additional rats inhaled 318 (range, 317.9-318.9) ppm 2,3-pentanedione for 6 hours and were sacrificed 0 to 2, 12 to 14, or 18 to 20 hours after exposure. Respiratory epithelial injury in the upper nose involved both apoptosis and necrosis, which progressed through 12 to 14 hours after exposure. Olfactory neuroepithelial injury included loss of olfactory neurons that showed reduced expression of the 2,3-pentanedione-metabolizing enzyme, dicarbonyl/L-xylulose reductase, relative to sustentacular cells. Caspase 3 activation occasionally involved olfactory nerve bundles that synapse in the olfactory bulb (OB). An additional group of rats inhaling 270 ppm 2,3-pentanedione for 6 hours 41 minutes showed increased expression of IL-6 and nitric oxide synthase-2 and decreased expression of vascular endothelial growth factor A in the OB, striatum, hippocampus, and cerebellum using real-time PCR. Claudin-1 expression increased in the OB and striatum. In other experiment, male and female rats and mice were exposed to 0, 50, 100, or 200 ppm 2,3-pentanedione 6 hr/d, 5 d/wk for up to 2 weeks. Bronchoalveolar lavage fluid (BALF) was collected after 1, 3, 5, and 10 exposures, and histopathology was evaluated after 12 exposures. MCP-1, MCP-3, CRP, FGF-9, fibrinogen, and OSM were increased 2- to 9-fold in BALF of rats exposed for 5 and 10 days to 200 ppm. In mice, only fibrinogen was increased after 5 exposures to 200 ppm. The epithelium lining the respiratory tract was the site of toxicity in all mice and rats exposed to 200 ppm. Significantly, 2,3-pentanedione also caused both intraluminal and intramural fibrotic airway lesions in rats. In third experiment, rats exposed to 150 or 200 ppm 2,3-pentanedione developed bronchial fibrosis. In mice, 2,3-pentanedione was not a dermal irritant when tested at concentrations up to 50%. However, concentration-dependent increases in lymphocyte proliferation were observed following exposure to 2,3-pentanedione in mice.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
皮肤致敏剂 - 一种可以诱导皮肤产生过敏反应的制剂。
Skin Sensitizer - An agent that can induce an allergic reaction in the skin.
来源:Haz-Map, Information on Hazardous Chemicals and Occupational Diseases
毒理性
/SRP:/ 紧急急救:确保已经进行了充分的中毒物清除。如果患者停止呼吸,开始人工呼吸,最好使用需求阀复苏器、袋阀面罩装置或口袋面罩,按训练操作。如有必要,执行心肺复苏。立即用缓慢流动的水冲洗受污染的眼睛。不要催吐。如果发生呕吐,让患者前倾或置于左侧卧位(如果可能的话,头部向下)以保持呼吸道畅通,防止吸入性肺炎。保持患者安静,维持正常体温。寻求医疗帮助。
/SRP:/ Immediate First Aid: Ensure that adequate decontamination has been carried out. If patient is not breathing, start artificial respiration, preferably with a demand-valve resuscitator, bag-valve-mask device, or pocket mask, as trained. Perform CPR if necessary. Immediately flush contaminated eyes with gently flowing water. Do not induce vomiting. If vomiting occurs, lean patient forward or place on left side (head-down position, if possible) to maintain an open airway and prevent aspiration. Keep patient quiet and maintain normal body temperature. Obtain medical attention.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 基本治疗:建立专利气道(如有需要,使用口咽或鼻咽气道)。必要时进行吸痰。观察呼吸不足的迹象,必要时协助通气。通过非重复呼吸面罩以10至15升/分钟的速度给予氧气。监测肺水肿,并在必要时治疗……。对于污染,立即用水冲洗眼睛。在转运过程中,用0.9%的生理盐水(NS)连续冲洗每只眼睛……。不要使用催吐剂。对于摄入,如果患者能吞咽、有强烈的咳嗽反射且不流口水,则用水冲洗口腔,并给予5毫升/千克,最多200毫升的水进行稀释。给予活性炭……。/酮体及其相关化合物/
/SRP:/ Basic treatment: Establish a patent airway (oropharyngeal or nasopharyngeal airway, if needed). Suction if necessary. Watch for signs of respiratory insufficiency and assist ventilations if necessary. Administer oxygen by nonrebreather mask at 10 to 15 L/min. Monitor for pulmonary edema and treat if necessary ... . For contamination, flush eyes immediately with water. Irrigate each eye continuously with 0.9% saline (NS) during transport ... . Do not use emetics. For ingestion, rinse mouth and administer 5 mL/kg up to 200 mL of water for dilution if the patient can swallow, has a strong gag reflex, and does not drool. Administer activated charcoal ... . /Ketones and related compounds/
来源:Hazardous Substances Data Bank (HSDB)
毒理性
/SRP:/ 高级治疗:对于无意识、严重肺水肿或严重呼吸困难的病人,考虑进行口咽或鼻咽气管插管以控制气道。使用气囊阀面罩装置的正压通气技术可能有益。考虑使用药物治疗肺水肿……。对于严重的支气管痉挛,考虑给予β激动剂,如沙丁胺醇……。监测心率和必要时治疗心律失常……。开始静脉输注D5W /SRP: "保持开放",最小流量/。如果出现低血容量的迹象,使用0.9%生理盐水(NS)或乳酸林格氏液(LR)。对于伴有低血容量迹象的低血压,谨慎给予液体。注意液体过载的迹象……。使用丙美卡因氢氯化物协助眼部冲洗……。/酮体及相关化合物/
/SRP:/ Advanced treatment: Consider orotracheal or nasotracheal intubation for airway control in the patient who is unconscious, has severe pulmonary edema, or is in severe respiratory distress. Positive-pressure ventilation techniques with a bag-valve-mask device may be beneficial. Consider drug therapy for pulmonary edema ... . Consider administering a beta agonist such as albuterol for severe bronchospasm ... . Monitor cardiac rhythm and treat arrhythmias if necessary ... . Start IV administration of D5W /SRP: "To keep open", minimal flow rate/. Use 0.9% saline (NS) or lactated Ringer's (LR) if signs of hypovolemia are present. For hypotension with signs of hypovolemia, administer fluid cautiously. Watch for signs of fluid overload ... . Use proparacaine hydrochloride to assist eye irrigation ... . /Ketones and related compounds/
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:F
-
安全说明:S16,S26,S36,S37/39
-
危险类别码:R36/37/38,R11
-
WGK Germany:1
-
海关编码:2914190090
-
危险品运输编号:UN 1224 3/PG 2
-
危险类别:3
-
RTECS号:SA1850000
-
包装等级:II
-
危险性防范说明:P210,P305+P351+P338
-
危险性描述:H225,H315,H319
-
储存条件:贮存:将密器密封后,储存在密封的主藏器中,并放置于阴凉、干燥处。
SDS
| Name: | 2 3-Pentanedione 97% Material Safety Data Sheet |
| Synonym: | Acetylpropiony |
| CAS: | 600-14-6 |
Synonym:Acetylpropiony
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 600-14-6 | 2,3-Pentanedione | 97 | 209-984-8 |
Risk Phrases: 11
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Highly flammable.
Potential Health Effects
Eye:
May cause eye irritation and possible burns.
Skin:
Causes skin irritation and possible burns.
Ingestion:
May cause digestive tract disturbances.
Inhalation:
May cause respiratory tract irritation. Vapors may cause dizziness or suffocation.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Remove contaminated clothing and shoes.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Water may be ineffective. Do NOT use straight streams of water. Cool containers with flooding quantities of water until well after fire is out.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors. Water spray may reduce vapor but may not prevent ignition in closed spaces.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep away from heat, sparks and flame. Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a cool, dry, well-ventilated area away from incompatible substances. Refrigerator/flammables. Keep containers tightly closed.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 600-14-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 110.0 - 112.0 deg C
Freezing/Melting Point: -52 deg C
Autoignition Temperature: Not available.
Flash Point: 18 deg C ( 64.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water: partially soluble
Specific Gravity/Density: .9570g/cm3
Molecular Formula: C5H8O2
Molecular Weight: 100.12
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, carbon dioxide, acrid smoke and fumes.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 600-14-6: SA1850000 LD50/LC50:
CAS# 600-14-6: Draize test, rabbit, skin: 500 mg/24H Moderate; Oral, rat: LD50 = 3 gm/kg; Skin, rabbit: LD50 = >2500 mg/kg.
Skin, Rabbitt:LD50 = >2500 Carcinogenicity:
2,3-Pentanedione - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: II
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.2
UN Number: 1993
Packing Group: II
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: II
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: F
Risk Phrases:
R 11 Highly flammable.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 33 Take precautionary measures against static
discharges.
S 50A Do not mix with acids.
WGK (Water Danger/Protection)
CAS# 600-14-6: 1
Canada
CAS# 600-14-6 is listed on Canada's DSL List.
CAS# 600-14-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 600-14-6 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2,3-戊二酮为黄绿色油状液体,具有甜白脱、奶油、焦糖香气,并带有坚果底香。其微溶于水,易溶于乙醇等有机溶剂,能发生二聚、氧化、还原、加成、缩合反应。可被高锰酸钾分解,与氢氧化钠作用转化为α-羟基酸。
用途 2,3-戊二酮可用作食品香精的香料、明胶硬化剂和相片的黏结剂等。我国GB 2760-86规定其为允许使用的食用香料,主要用于配制巧克力和奶油型香精。天然存在于咖啡、啤酒、老姆酒、威士忌、红葡萄酒、白葡萄酒中,也存在于芬兰松的精油中。
含量分析 按醛和酮测定法(OT-7)中的方法一(羟胺法)测定,所取试样量为400mg。计算中的当量因子(e)取25.03。或按气相色谱法(GT-10-4)中用非极性柱的方法测定。
毒性 GRAS(FEMA)。
使用限量 FEMA(mg/kg):软饮料0.60;冷饮3.3;糖果5.9;焙烤食品9.6;布丁类0.28;裱花层0.30。适度为限(FDA§172.515,2000)。
最大允许使用量与残留标准 根据GB 2760--1996规定,2,3-戊二酮作为食品用香料的最大允许使用量和最大允许残留量应不超过GB 2760中的标准。主要用于配制巧克力和奶油型香精。
生产方法 天然存在于芬兰松等精油中。人工合成可在盐酸羟胺存在下,于氮气保护下,用过量亚硝酸钠和稀盐酸使甲基丙酮氧化而成。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,4-己二酮 3,4-hexanedione 4437-51-8 C6H10O2 114.144 2-戊酮 2-Pentanone 107-87-9 C5H10O 86.1338 3-戊酮 pentan-3-one 96-22-0 C5H10O 86.1338 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 庚烷-3,4-二酮 heptane-3,4-dione 13706-89-3 C7H12O2 128.171 2-戊酮 2-Pentanone 107-87-9 C5H10O 86.1338 3-戊酮 pentan-3-one 96-22-0 C5H10O 86.1338 辛烷-3,4-二酮 octane-3,4-dione 3457-39-4 C8H14O2 142.198
反应信息
-
作为反应物:参考文献:名称:Synthesis for 7-alkylamino-3-methylpyrazolo [4,3-d]pyrimidines摘要:公开了一种改进的合成方法,用于合成已知为强效细胞分裂素拮抗剂的7-烷基氨基-3-甲基吡唑并[4,3-d]嘧啶。公开号:US04282361A1
-
作为产物:描述:参考文献:名称:α-二酮,α,α-二烷氧基酮和α-烷氧基-α-亚磺酰基化酮的区域专一性合成摘要:通过汞诱导的区域特异性形成的α-氯代-α-(烷硫基)酮的溶剂化反应,开发了一种方便的合成α-二酮及其单保护缩醛,即α-酮缩醛的方法。类似地,当α-氯-α-亚磺酰基化的酮与碱性醇介质反应时,形成α-烷氧基-α-亚磺酰基化的酮。α-烷氧基-α-亚磺酰化的酮本身可以转化为α-二酮或α-酮缩醛,然后在无水条件下将其水解为相应的α-二酮。DOI:10.1016/s0040-4020(00)00604-9
-
作为试剂:描述:2-溴苯甲酸 在 2,3-戊二酮 、 copper(ll) bromide sodium carbonate 、 水 、 盐酸 作用下, 以 水 为溶剂, 反应 17.0h, 以94%的产率得到水杨酸参考文献:名称:Process for the synthesis of hydroxy aromatic acids摘要:在含有铜源和配位于铜的配体的反应混合物中,从卤代芳香酸中高产率和高纯度(> 95%)地产生羟基芳香酸。公开号:US07351856B1
文献信息
-
Synthesis and Characterisation of Indium(III) Bis-Thiosemicarbazone Complexes: 18F Incorporation for PET Imaging作者:Taracad K. Venkatachalam、Paul V. Bernhardt、Gregory K. Pierens、Damion H. R. Stimson、Rajiv Bhalla、David C. ReutensDOI:10.1071/ch18559日期:——coordination sphere in all indium complexes. In some complexes, an intermolecular hydrogen bond was present between the chlorine atom and an NH group. Three different indium chlorido complexes were converted into the corresponding fluorido-derivative by a simple halide exchange method using K18F. These novel complexes, containing the positron emitting isotope 18F, may have potential applications in positron
-
Identification of differential anti-neoplastic activity of copper bis(thiosemicarbazones) that is mediated by intracellular reactive oxygen species generation and lysosomal membrane permeabilization作者:Christian Stefani、Zaynab Al-Eisawi、Patric J. Jansson、Danuta S. Kalinowski、Des R. RichardsonDOI:10.1016/j.jinorgbio.2015.08.010日期:2015.11and disubstituted bis(thiosemicarbazones). This alkyl substitution pattern governed their: (1) CuII/I redox potentials; (2) ability to induce cellular 64Cu release; (3) lipophilicity; and (4) anti-proliferative activity. The potent anti-cancer Cu complex of the unsubstituted bis(thiosemicarbazone) analog, glyoxal bis(4-methyl-3-thiosemicarbazone) (GTSM), generated intracellular reactive oxygen species双(硫代半脲)及其铜(Cu)配合物具有独特的抗肿瘤特性。但是,它们的作用机理仍不清楚。我们检查了十二个双(硫代半氨基甲酮)的结构-活性关系,以阐明有关其抗癌功效的因素。重要的是,在配体主链的二亚胺位置上的烷基取代产生两个不同的基团,即未取代/单取代和二取代的双(硫代半咔唑酮)。这种烷基取代模式控制着它们:(1)Cu II / I氧化还原电势;(2)诱导细胞64的能力铜释放;(3)亲脂性;(4)抗增殖活性。未取代的双(硫代半碳zone酮)类似物乙二醛双(4-甲基-3-硫代半碳zone酮)(GTSM)的有效抗癌铜络合物可生成细胞内活性氧(ROS),并通过非螯合的铜螯合作用而减弱有毒的铜螯合剂四硫代钼酸盐和抗氧化剂N-乙酰-1-半胱氨酸。荧光显微镜显示,Cu(GTSM)的抗癌活性部分归因于溶酶体膜通透性(LMP)。这项研究首次凸显了ROS和LMP在双(硫代半脲酮)的抗癌活性中的作用。
-
Copper complexes with dissymmetrically substituted bis(thiosemicarbazone) ligands as a basis for PET radiopharmaceuticals: control of redox potential and lipophilicity作者:Oliver C. Brown、Julia Baguña Torres、Katherine B. Holt、Philip J. Blower、Michael J. WentDOI:10.1039/c7dt02008b日期:——synthesis of a library of new dissymmetrically substituted bis(thiosemicarbazone) ligands by controlling the condensation reactions between dicarbonyl compounds and 4-substituted-3-thiosemicarbazides or using acetal protection. Copper complexes of the new ligands have been prepared by reaction with copper acetate or via transmetallation of the corresponding zinc complexes, which are convenient precursors for
-
Palladium-Catalyzed Direct and Specific C-7 Acylation of Indolines with 1,2-Diketones作者:Guilin Xie、Yuhan Zhao、Changqun Cai、Guo-Jun Deng、Hang GongDOI:10.1021/acs.orglett.0c03922日期:2021.1.15indole framework and/or realize indole modification. Nevertheless, the direct selective functionalization on the benzenoid core must overcome the high activity of the C-3 position and still remains highly challenging. Herein, a palladium-catalyzed direct and specific C-7 acylation of indolines in the presence of an easily removed directing group was developed. This strategy usually is considered as a practical
-
[EN] THIAZOLOPYRIMIDINONES AS MODULATORS OF NMDA RECEPTOR ACTIVITY<br/>[FR] THIAZOLOPYRIMIDINONES EN TANT QUE MODULATEURS DE L'ACTIVITÉ DU RÉCEPTEUR NMDA申请人:HOFFMANN LA ROCHE公开号:WO2015052226A1公开(公告)日:2015-04-16The present invention relates to certain thiazolopyrimidinone compounds for use in modulating NMDA receptor activity, pharmaceutical compositions comprising such compounds and methods of treating neurological and psychiatric conditions.
表征谱图
-
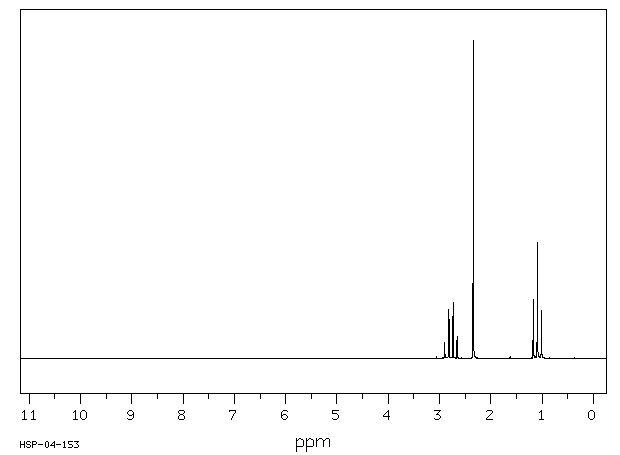
氢谱1HNMR
-
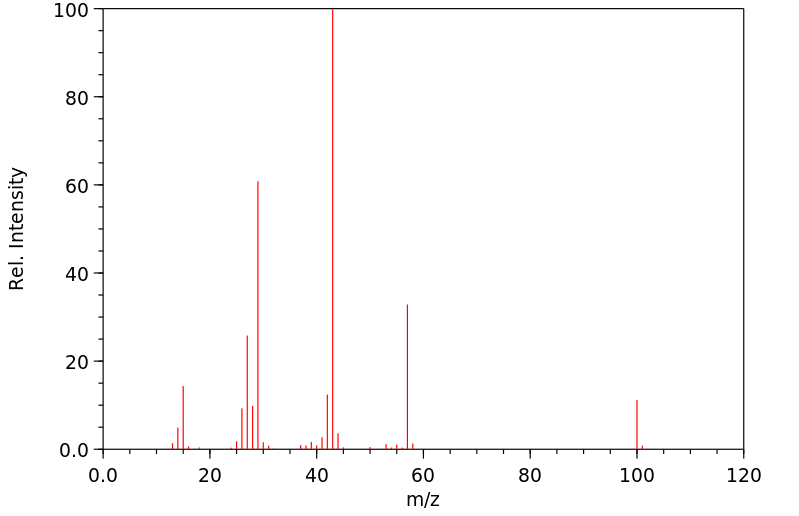
质谱MS
-
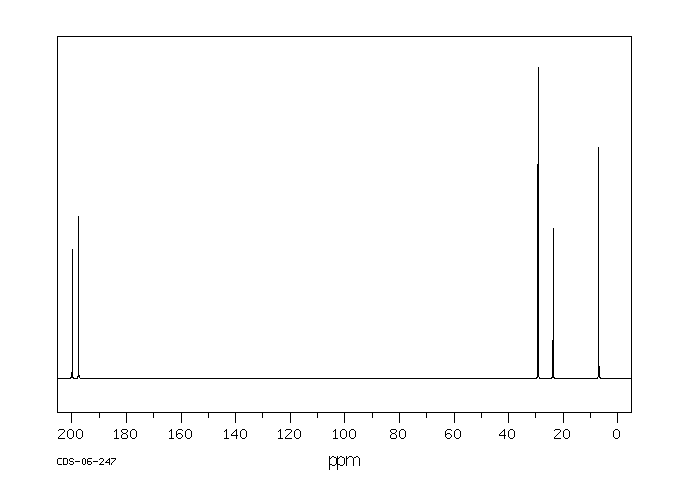
碳谱13CNMR
-
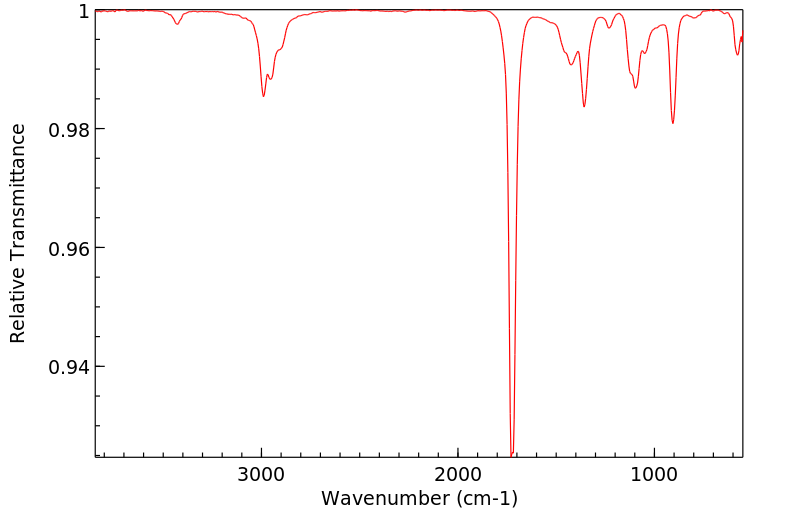
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷