6-氨基吲唑 | 6967-12-0
中文名称
6-氨基吲唑
中文别名
6-氨基-1H-吲唑;1H-吲哚唑-6-胺
英文名称
6-amino-1H-indazole
英文别名
6-Aminoindazole;1H-indazol-6-amine
CAS
6967-12-0
化学式
C7H7N3
mdl
MFCD00005696
分子量
133.153
InChiKey
KEJFADGISRFLFO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204-206 °C (dec.) (lit.)
-
沸点:376.6±15.0 °C(Predicted)
-
密度:1.367±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:10
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:54.7
-
氢给体数:2
-
氢受体数:2
安全信息
-
安全说明:S26
-
WGK Germany:3
-
海关编码:2933990090
-
危险品标志:Xn
-
危险类别码:R22,R36/37/38
-
RTECS号:NK7712000
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302,H315,H319,H332,H335
-
储存条件:| 室温 |
SDS
| Name: | 6-Aminoindazole 97+% Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 6967-12-0 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 6967-12-0 | 6-Aminoindazole | >97 | 230-177-1 |
Risk Phrases: 22
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Harmful if swallowed.The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 6967-12-0: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: slightly brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 204.00 - 206.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C7H7N3
Molecular Weight: 133.0691
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 6967-12-0: NK7712000 LD50/LC50:
CAS# 6967-12-0: Oral, mouse: LD50 = 2 gm/kg.
Carcinogenicity:
6-Aminoindazole - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XN
Risk Phrases:
R 22 Harmful if swallowed.
Safety Phrases:
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 6967-12-0: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 6967-12-0 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 6967-12-0 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
6-氨基吲唑是一种有机中间体,文献报道它可用于制备抗肿瘤化合物 CYY-260。
制备6-氨基吲唑可由2-氨基-4-硝基甲苯先关环制备成6-硝基吲唑,然后还原硝基得到6-氨基吲唑。
具体步骤如下:
- 取2-氨基-4-硝基甲苯(5.00g,32.9mmol),溶解于冰醋酸(235mL)中。
- 在搅拌下,一次性加入亚硝酸钠(2.50g,36.2mmol)的水(6mL)溶液,保持体系温度不高于20℃。
- 搅拌反应24小时后,将冰醋酸减压旋蒸出大部分,加大量水,析出固体,抽滤得到固体,用水洗涤并真空干燥,最终获得6-硝基吲唑。
- 取6-硝基吲唑(1.00g,6.10mmol),加入10%Pd/C(0.200g)于甲醇(60mL)中,再加入甲酸胺(3.86g,61.0mmol)。
- 搅拌下加热至回流反应约50分钟。通过TLC监测确保反应完全。
- 过滤除去催化剂,并用甲醇(30mL)洗涤后减压回收溶剂,获得初产物6-氨基吲唑。
6-氨基吲唑为淡棕色固体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-硝基吲唑 6-nitroindazole 7597-18-4 C7H5N3O2 163.136 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 6-肼基-1H-吲唑 6-Hydrazinyl-1H-indazole 72372-66-8 C7H8N4 148.167 —— 6-(methylamino)indazole —— C8H9N3 147.18 5-叠氮基-1H-吲唑 5-azido-1H-indazole 20377-00-8 C7H5N5 159.15 —— N-ethylideneindazol-6-amine 88369-86-2 C9H9N3 159.191 —— 6-ethylaminoindazole 88369-87-3 C9H11N3 161.206 —— 6-formylamino-1H-indazole 77929-35-2 C8H7N3O 161.163 N-(1-甲基乙基)-1H-吲唑-6-胺 N-isopropyl-1H-indazol-6-amine 1152873-01-2 C10H13N3 175.233 N-(1H-吲唑-6-基)硫脲 (1H-indazol-6-yl)-thiourea 99055-55-7 C8H8N4S 192.244 —— N-(1H-indazol-6-yl)acetamide 13551-95-6 C9H9N3O 175.19 —— 1(2)H-indazole-6,7-diyldiamine 39761-90-5 C7H8N4 148.167 吲唑 benzimidazole 271-44-3 C7H6N2 118.138 —— N-cyclopentyl-1H-indazol-6-amine 1157521-65-7 C12H15N3 201.271 —— 6-(3-methyl-thioureido)-1H-indazole 451514-78-6 C9H10N4S 206.271 —— methyl (1H-indazol-6-yl)-dithiocarbamate 1046454-12-9 C9H9N3S2 223.323 —— N-benzyl-1H-indazol-6-amine —— C14H13N3 223.277 —— 2-chloro-N-(1H-indazol-6-yl)acetamide —— C9H8ClN3O 209.63 —— N-[(4-aminophenyl)methyl]-1H-indazol-6-amine 629630-31-5 C14H14N4 238.292 6-(哌嗪-1-基)-1H-吲唑 6-(piperazin-1-yl)-1H-indazole 763910-07-2 C11H14N4 202.259 —— 6-(3-ethyl-thioureido)-1H-indazole 454188-51-3 C10H12N4S 220.298 —— ethyl (1H-indazol-6-yl)-dithiocarbamate 1046454-13-0 C10H11N3S2 237.349 —— 2-[(E)-Hydroxyimino]-N-(1H-indazol-6-yl)-acetamide 73907-93-4 C9H8N4O2 204.188 —— 6-(isonitrosoacetamido)indazole 73907-93-4 C9H8N4O2 204.188 7-溴-1H-吲唑-6-胺 7-bromo-1H-indazol-6-amine 139502-26-4 C7H6BrN3 212.049 —— 6-[3-(2-chloroethyl)ureido]indazole 161194-49-6 C10H11ClN4O 238.677 —— (Z)-4-(1H-indazol-6-ylamino)-4-oxobut-2-enoic acid —— C11H9N3O3 231.211 —— ethyl 2-[(1H-indazol-6-yl)-hydrazono]-propionate 933474-32-9 C12H14N4O2 246.269 6-羟基吲唑 1H-indazol-6-ol 23244-88-4 C7H6N2O 134.137 —— 1-[(methyloxy)methyl]-1H-indazol-6-amine 123177-52-6 C9H11N3O 177.206 —— ethyl (E)-3-(1H-indazol-6-ylamino)but-2-enoate —— C13H15N3O2 245.281 —— Ethyl-3-(6-Indazolylamino)crotonat 54996-75-7 C13H15N3O2 245.281 —— 6-(3-benzyl-thioureido)-1H-indazole 502856-27-1 C15H14N4S 282.369 —— N-(1H-indazol-6-yl)cyclohexanecarboxamide —— C14H17N3O 243.308 —— 1-(2-cyclohexylethyl)-3-(1H-indazol-6-yl)urea 1426152-37-5 C16H22N4O 286.377 —— ethyl 3-(1H-indazol-6-ylamino)-3-oxopropanoate 105397-85-1 C12H13N3O3 247.254 6-碘-1H-吲唑 6-Iodo-1H-indazole 261953-36-0 C7H5IN2 244.035 6-溴吲唑 6-bromo-1H-indazole 79762-54-2 C7H5BrN2 197.034 3-(6-氨基吲唑-1-基)-丙腈 3-(6-amino-indazol-1-yl)-propionitrile 99072-66-9 C10H10N4 186.216 6-氟(1H)吲唑 6-fluoro-1H-indazole 348-25-4 C7H5FN2 136.129 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:WO2006/71940摘要:公开号:
-
作为产物:参考文献:名称:Witt; Noelting; Grandmougin, Chemische Berichte, 1890, vol. 23, p. 3642摘要:DOI:
-
作为试剂:描述:4-硝基苯甲酰氯 、 5-氨基-1-(2-吡啶基)-1H-吡唑-4-羧酸乙酯 在 6-氨基吲唑 作用下, 以 乙腈 为溶剂, 反应 8.0h, 以43%的产率得到ethyl 5-(4-nitrobenzamido)-1-(pyridin-2-yl)-1H-pyrazole-4-carboxylate参考文献:名称:带有吡唑或吲唑核的新型4-(3-苯基丙酰胺基),4-(2-苯氧基乙酰胺基)和4-(肉桂酰胺基)取代的苯甲酰胺:合成,生物学评估和作用机理。摘要:基于已知化合物干扰p53途径和我们先前合成的苯甲酰胺的一些共同结构特征,我们合成了新的5-(4-取代的苯甲酰胺)-1-苯基-1H-吡唑-4-羧酸乙酯26a-c,5-乙基(4-取代的苯甲酰胺)-1-(吡啶-2-基)-1H-吡唑-4-羧酸酯27a-c和N-(1H-吲唑-6-基)-4-取代的苯甲酰胺31a,b苯甲酰胺基的4位是2-苯基丙酰胺基或2-苯氧基乙酰胺基或肉桂酰胺基。评估抗人肺癌H292细胞抗增殖活性的初步测试突出了化合物26c如何表现出最佳活性。因此,选择了这最后一个用于进一步研究,目的是寻找作用机理。DOI:10.1016/j.bioorg.2018.10.055
文献信息
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] PYRROLOTRIAZINES AS ALK AND JAK2 INHIBITORS<br/>[FR] PYRROLOTRIAZINES EN TANT QU'INHIBITEURS D'ALK ET DE JAK2申请人:CEPHALON INC公开号:WO2010071885A1公开(公告)日:2010-06-24The present invention provides a compound of formula (I) or a salt form thereof, wherein Q1, Q2, Q3, and Q4 are as defined herein. The compound of formula (I) has ALK and/or JAK2 inhibitory activity, and may be used to treat proliferative disorders.本发明提供了一种式(I)的化合物或其盐形式,其中Q1、Q2、Q3和Q4如本文所定义。式(I)的化合物具有ALK和/或JAK2抑制活性,并可用于治疗增殖性疾病。
-
COMPOSITIONS FOR TREATMENT OF CYSTIC FIBROSIS AND OTHER CHRONIC DISEASES申请人:Van Goor Fredrick F.公开号:US20110098311A1公开(公告)日:2011-04-28The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
[EN] SUBSTITUTED TETRAHYDROISOQUINOLINE COMPOUNDS AS FACTOR XIA INHIBITORS<br/>[FR] COMPOSÉS TÉTRAHYDROISOQUINOLINES SUBSTITUÉS EN TANT QU'INHIBITEURS DU FACTEUR XIA申请人:BRISTOL MYERS SQUIBB CO公开号:WO2013055984A1公开(公告)日:2013-04-18The present invention provides compounds of Formula (I): or stereoisomers, pharmaceutically acceptable salts thereof, wherein all of the variables are as defined herein. These compounds are inhibitors of factor XIa and/or plasma kallikrein which may be used as medicaments.本发明提供了化合物的公式(I):或其立体异构体,以及其药学上可接受的盐,其中所有变量如本文所定义。这些化合物是XIa因子和/或血浆激肽酶的抑制剂,可用作药物。
-
Anti-Viral Compounds申请人:DeGoey David A.公开号:US20100317568A1公开(公告)日:2010-12-16Compounds effective in inhibiting replication of Hepatitis C virus (“HCV”) are described. This invention also relates to processes of making such compounds, compositions comprising such compounds, and methods of using such compounds to treat HCV infection.描述了一种有效抑制丙型肝炎病毒(“HCV”)复制的化合物。本发明还涉及制备这种化合物的方法、包含这种化合物的组合物,以及使用这种化合物治疗HCV感染的方法。
表征谱图
-
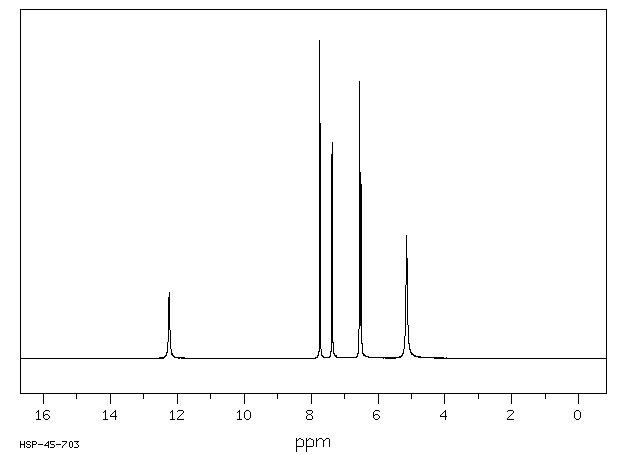
氢谱1HNMR
-
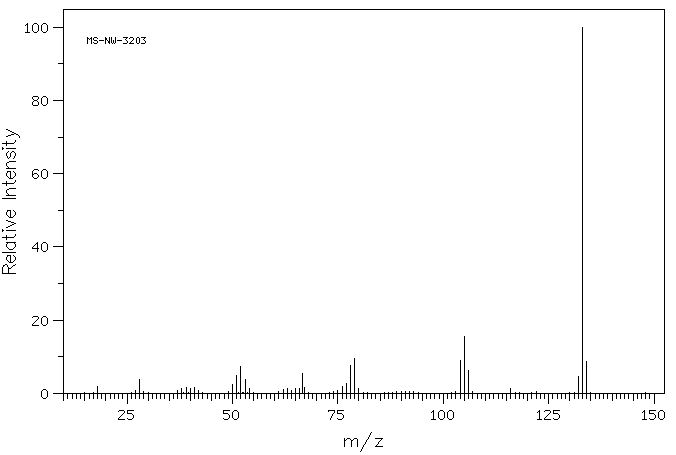
质谱MS
-
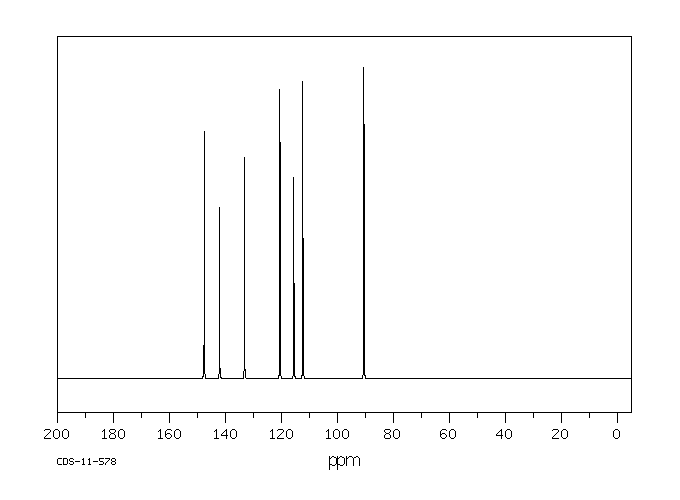
碳谱13CNMR
-
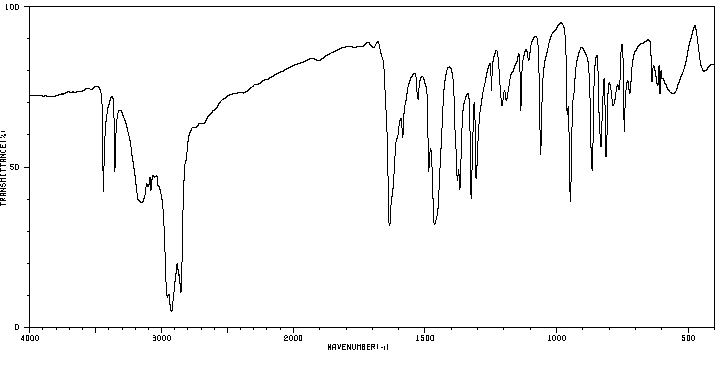
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(乙基N-(1H-吲唑-3-基羰基)ethanehydrazonoate)
(2R,6S)-2,6-二甲基-4-[6-[5-(1-(甲基环丙基)氧基]-1H-吲唑-3-基]嘧啶-4-基]吗啉
顺式-八氢-2-甲基-吡咯并[3,4-c]吡咯
陀尼达安
阿西替尼杂质
阿布查米卡代谢物M4
达泽达明
苯达扎克钠
苯甲酸,5-(溴甲基)-2-硝基-,甲基酯
苯乙胺杂质8
苯丙酰胺,b-氨基-3-乙氧基-
苄达酸钠盐
苄达酸
苄达明 N-氧化物
苄达明
苄胺N-氧化物马来酸氢盐
苄基-(3-氯-5-硝基-1H-吲唑-7-基)-胺
盐酸苄达明
1-(2,4-二氯苄基)-1H-吲唑-3-碳酰肼
甲基7-氨基-1H-吲唑-3-羧酸酯
甲基5-溴-1-(四氢-2H-吡喃-2-基)-1H-吲唑-3-甲酸基酯
甲基4-碘-1H-吲唑-6-羧酸
甲基4-氨基-1-乙基-1H-吲唑-6-羧酸酯
甲基-6-硝基-1H-吲唑-3-羧酸
甲基 1H-吲唑-7-羧酸盐酸盐
水杨酸化合物与3-[(1-苄基-1H-吲唑-3-基)氧基]-N,N-二甲基丙基胺(1:1)
氯尼达明
格拉斯琼杂质C
格拉司琼相关物质C
格拉司琼相关物质A
格拉司琼氮氧化物
帕唑帕尼相关化合物2
帕唑帕尼杂质57
希尼达明
宾达利
奈米利塞
奈拉米诺
因旦尼定
四溴甲基-1-甲基吲唑
哌啶,2-乙基-1-(3-苯基-2-丙炔-1-基)-
吲熟酯
吲唑-6-硼酸
吲唑-5-甲酸盐酸盐
吲唑-5-甲酸甲酯
吲唑-5-甲腈
吲唑-4-硼酸盐酸盐
吲唑-4-乙酸
吲唑-3-羧酸乙脂
吲唑-3-羧酸
吲唑-3-乙酸