5-硝基噻吩-2-甲醛 | 4521-33-9
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:75-77 °C (lit.)
-
沸点:118-120 °C(Press: 3-4 Torr)
-
密度:1.534±0.06 g/cm3(Predicted)
-
溶解度:丙酮:可溶1%,澄清,黄色
-
稳定性/保质期:
在常温常压下保持稳定。
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:10
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:91.1
-
氢给体数:0
-
氢受体数:4
安全信息
-
安全说明:S26
-
危险品运输编号:NONH for all modes of transport
-
WGK Germany:3
-
海关编码:2934999090
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
储存条件:请将药品存放在避光、通风且干燥的地方,并密封保存。
SDS
: 5-Nitro-2-thiophenECarboxaldehyde
化学品俗名或商品名
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别2)
眼刺激 (类别2A)
特异性靶器官系统毒性(一次接触) (类别3)
2.2 GHS 标记要素,包括预防性的陈述
危害类型象形图
信号词 警告
危险申明
H315 造成皮肤刺激。
H319 造成严重眼刺激。
H335 可能引起呼吸道刺激。
警告申明
预防
P261 避免吸入粉尘/ 烟/ 气体/ 烟雾/ 蒸汽/ 喷雾。
P264 操作后彻底清洁皮肤。
P271 只能在室外或通风良好之处使用。
P280 穿戴防护手套/ 眼保护罩/ 面部保护罩。
措施
P302 + P352 如果在皮肤上: 用大量肥皂和水淋洗。
P304 + P340 如果吸入: 将患者移到新鲜空气处休息,并保持呼吸舒畅的姿势。
P305 + P351 + P338 如进入眼睛:用水小心清洗几分钟。如戴隐形眼镜并可方便地取出,取出
隐形眼镜。继续冲洗。
P312 如感觉不适,呼救解毒中心或医生。
P321 具体治疗(见本标签上提供的急救指导)。
P332 + P313 如发生皮肤刺激:求医/ 就诊。
P337 + P313 如仍觉眼睛刺激:求医/ 就诊。
P362 脱掉沾染的衣服,清洗后方可重新使用。
储存
P403 + P233 存放于通风良的地方。 保持容器密闭。
P405 存放处须加锁。
处理
P501 将内容物/ 容器处理到得到批准的废物处理厂。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C5H3NO3S
分子式
: 157.15 g/mol
分子量
成分 浓度
5-Nitrothiophene-2-carbaldehyde
-
化学文摘编号(CAS No.) 4521-33-9
EC-编号 224-850-9
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
如果吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
在皮肤接触的情况下
用肥皂和大量的水冲洗。 请教医生。
在眼睛接触的情况下
用大量水彻底冲洗至少15分钟并请教医生。
如果误服
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 最重要的症状和影响,急性的和滞后的
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物, 氮氧化物, 硫氧化物
5.3 救火人员的预防
如必要的话,戴自给式呼吸器去救火。
5.4 进一步的信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。
将人员撤离到安全区域。 避免吸入粉尘。
6.2 环境预防措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放在合适的封闭的处理容器内。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
避免接触皮肤和眼睛。 防止粉尘和气溶胶生成。
在有粉尘生成的地方,提供合适的排风设备。一般性的防火保护措施。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制/个体防护
8.1 控制参数
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
根据工业卫生和安全使用规则来操作。 休息以前和工作结束时洗手。
人身保护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
防渗透的衣服, 防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
如须暴露于有害环境中,请使用P95型(美国)或P1型(欧盟 英国
143)防微粒呼吸器。如需更高级别防护,请使用OV/AG/P99型(美国)或ABEK-P2型 (欧盟 英国 143)
防毒罐。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 固体
颜色: 黄色
b) 气味
无数据资料
c) 气味临界值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/熔点范围: 75 - 77 °C - lit.
f) 起始沸点和沸程
无数据资料
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 可燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸气压
无数据资料
l) 相对蒸气密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) 辛醇/水分配系数的对数值
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 化学稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 避免接触的条件
无数据资料
10.5 不兼容的材料
强氧化剂, 强碱
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
无数据资料
皮肤腐蚀/刺激
无数据资料
严重眼损伤 / 眼刺激
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞诱变
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
吸入 - 可能引起呼吸道刺激。
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 造成皮肤刺激。
眼睛 造成严重眼刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: 无数据资料
模块 12. 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 生物积累的潜在可能性
无数据资料
12.4 土壤中的迁移
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。 联系专业的拥有废弃物处理执照的机构来处理此物质。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
污染了的包装物
作为未用过的产品弃置。
模块 14. 运输信息
14.1 UN编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 无危险货物
国际海运危规: 无危险货物
国际空运危规: 无危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别预防
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
噻吩类化合物具有抗菌消炎、抗病毒和解痉挛等药理学活性。研究它们与牛血清白蛋白(BSA)的作用对理解其药代动力学及开发新药具有重要意义。
5-硝基噻吩-2-甲醛是有机合成中的重要中间体,不仅可作为药物中间体应用于医药工业,还可用于合成一系列硝基噻吩类席夫碱。
制备将0.1 mol 5-氯-2-硝基苯甲醛、0.005 mol 乙酸钯和0.006 mol 1,10-菲罗啉溶解于20 mL N,N-二甲基甲酰胺中,随后滴加0.15 mmol 六氢吡啶。在60℃下搅拌24小时后,将反应液倒入水中进行萃取,再通过重结晶得到5-硝基噻吩-2-甲醛,产率为63%。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-氯甲基-5-硝基噻吩 2-(chloromethyl)-5-nitrothiophene 20898-86-6 C5H4ClNO2S 177.611 (5-硝基噻吩-2-基)甲醇 (5-nitrothiophen-2-yl)methanol 20898-85-5 C5H5NO3S 159.166 —— 2,2-dimethyl-1-(5'-nitro-2'-thienyl)-propane-1-one 83054-95-9 C9H11NO3S 213.257 —— (5'-nitro-2'-thienyl)methyl acetate 20898-83-3 C7H7NO4S 201.203 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 5-硝基-2-甲基噻吩 2-methyl-5-nitrothiophene 42297-94-9 C5H5NO2S 143.166 2-乙酰基-5-硝基噻吩 2-acetyl-5-nitrothiophene 39565-00-9 C6H5NO3S 171.177 5-硝基噻吩-2-羰酰氯 5-nitrothiophene-2-carbonyl chloride 39978-57-9 C5H2ClNO3S 191.595 5-硝基噻吩-2-甲酸 5-nitrothiophene-2-carboxylic acid 6317-37-9 C5H3NO4S 173.149 2-(溴甲基)-5-硝基-噻吩 2-(bromomethyl)-5-nitrothiophene 166887-84-9 C5H4BrNO2S 222.062 2-氯甲基-5-硝基噻吩 2-(chloromethyl)-5-nitrothiophene 20898-86-6 C5H4ClNO2S 177.611 (5-硝基噻吩-2-基)甲醇 (5-nitrothiophen-2-yl)methanol 20898-85-5 C5H5NO3S 159.166 5-硝基噻酚-2-甲腈 5-nitro-2-thienyl cyanide 16689-02-4 C5H2N2O2S 154.149 5-硝基-噻吩-2-甲酸甲酯 methyl 5-nitrothiophene-2-carboxylate 5832-01-9 C6H5NO4S 187.176 —— 5-nitrothiophene-2-carbaldehyde oxime 6030-18-8 C5H4N2O3S 172.164 —— 5-nitrothiophene-2-aldoxime 104416-19-5 C5H4N2O3S 172.164 —— 5-nitro-thiophene-2-carboxylic acid allylamide 133628-36-1 C8H8N2O3S 212.229 —— (2E)-3-(5-nitro-2-thienyl)-2-propenal 62391-19-9 C7H5NO3S 183.188 3-(5-硝基噻吩-2-基)丙-2-烯醛 β-(5-nitro-2-thienyl)acrylaldehyde 62391-19-9 C7H5NO3S 183.188 —— tert-butyl 5-nitrothiophene-2-carboxylate 62224-28-6 C9H11NO4S 229.257 —— (3E)-4-(5-nitro-2-thienyl)-3-buten-2-one 57559-00-9 C8H7NO3S 197.214 —— 3t-(5-nitro-[2]thienyl)-acrylic acid amide 98550-02-8 C7H6N2O3S 198.202 —— 3-(5-nitrothiophen-2-yl)propenoic acid 17163-22-3 C7H5NO4S 199.187 3-(5-硝基-2-噻吩)丙烯酸 3-(5-nitro-2-thienyl)-2-propenoic acid 50868-70-7 C7H5NO4S 199.187 —— (E)-3-(5-nitrothiophen-2-yl)acryloyl chloride 55785-89-2 C7H4ClNO3S 217.633 —— 2-(5-nitrothiophen-2-ylmethylene)malononitrile 84653-70-3 C8H3N3O2S 205.197 —— 1-(5-nitrothiophen-2-yl)ethan-1-ol 74786-64-4 C6H7NO3S 173.192 —— 2-nitro-5-(2-nitro-vinyl)-thiophene 34210-00-9 C6H4N2O4S 200.175 —— 5-nitro-2-thiophene semicarbazone 6286-12-0 C6H6N4O3S 214.205 —— 2-((5-nitrothiophen-2-yl)methylene)hydrazinecarbothioamide 5351-83-7 C6H6N4O2S2 230.271 —— 5-nitro-2-thiophene thiosemicarbazone 5351-83-7 C6H6N4O2S2 230.271 —— 2-nitro-N-(oxiranylmethyl)thiophene-5-carboxamide 133628-37-2 C8H8N2O4S 228.229 —— 5-(dimethoxymethyl)-2-nitrothiophene 17375-68-7 C7H9NO4S 203.219 —— 2-nitro-5-benzylthiophene 37715-64-3 C11H9NO2S 219.264 —— (E)-1-(5-Nitrothiophen-2-yl)-N-phenylmethanimine 40619-46-3 C11H8N2O2S 232.263 —— methyl N-[(5-nitrothiophen-2-yl)methylideneamino]carbamate —— C7H7N3O4S 229.216 —— 1-[2-(5-nitrothienyl)methylene]-4-hydroxysemicarbazide 395639-36-8 C6H6N4O4S 230.204 —— 2-bromo-3ξ-(5-nitro-[2]thienyl)-acrylaldehyde 17164-74-8 C7H4BrNO3S 262.084 —— methanesulfonic acid (5-nitrothiophen-2-yl)methanol ester 946126-96-1 C6H7NO5S2 237.257 —— 5-nitro-thiophene-2-carbaldehyde phenylhydrazone 91093-51-5 C11H9N3O2S 247.277 —— N-(4-chlorobenzyl)-1-(5-nitrothiophen-2-yl)methanamine 1384516-10-2 C12H11ClN2O2S 282.751 —— 3-(5-nitro-thiophen-2-yl)-acrylic acid ethyl ester 17163-21-2 C9H9NO4S 227.241 —— N-(cyclohexylmethyl)-1-(5-nitrothiophen-2-yl)methanimine 78060-09-0 C12H16N2O2S 252.337 —— N-cyclohexyl-1-(5-nitrothiophen-2-yl)methanimine 69819-73-4 C11H14N2O2S 238.31 —— (5-nitro-thiophen-2-ylmethylene)-[1,2,4]triazol-4-yl-amine 31539-41-0 C7H5N5O2S 223.215 N-(4-溴苯基)-1-(5-硝基噻吩-2-基)甲亚胺 N-(5-nitro-2-thienomethylidene)-4-bromoaniline 64857-15-4 C11H7BrN2O2S 311.159 - 1
- 2
- 3
- 4
- 5
反应信息
-
作为反应物:描述:5-硝基噻吩-2-甲醛 在 sodium tetrahydroborate 作用下, 以 乙醇 为溶剂, 反应 5.0h, 以97.2%的产率得到(5-硝基噻吩-2-基)甲醇参考文献:名称:在压力驱动的流动反应器中使用二氧化硅负载的琼斯试剂对芳香醇进行清洁和选择性氧化摘要:通过利用连续流动反应器中获得的高表面积体积比,我们能够根据所使用的流速将一系列伯醇选择性地氧化为醛或羧酸,证明了在传统搅拌条件下无法实现的反应控制程度反应堆。DOI:10.1016/j.tetlet.2006.05.157
-
作为产物:描述:(5-硝基噻吩-2-基)甲醇 在 silica-supported Jones reagent 作用下, 以 二氯甲烷 为溶剂, 以99.8%的产率得到5-硝基噻吩-2-甲醛参考文献:名称:在压力驱动的流动反应器中使用二氧化硅负载的琼斯试剂对芳香醇进行清洁和选择性氧化摘要:通过利用连续流动反应器中获得的高表面积体积比,我们能够根据所使用的流速将一系列伯醇选择性地氧化为醛或羧酸,证明了在传统搅拌条件下无法实现的反应控制程度反应堆。DOI:10.1016/j.tetlet.2006.05.157
-
作为试剂:描述:1-(4-chlorophenyl)-2-methoxyethylene 以 5-硝基噻吩-2-甲醛 为溶剂, 生成 2-(4-chlorophenyl)-3-(5-nitro-thien-2-yl)acrolein参考文献:名称:Synergistic compositions摘要:本发明提供了一种包含苯并异噻唑啉衍生物和2-硝基呋喃或2-硝基噻吩衍生物的协同组合物。这些组合物可用作抗微生物剂。公开号:US03982007A1
文献信息
-
Synthesis and Biological Activity of Novel (E)-N’-(Substituted)-3,4,5-Trimethoxybenzohydrazide Analogs作者:Namala Rambabu、Bhavani Ram、Pramod Kumar Dubey、Bhavani Vasudha、Bhavani BalramDOI:10.13005/ojc/330126日期:2017.2.28Aspergillus niger and Candida albicans (Fungal strains). The results revealed that most of the hydrazone derivatives exhibited significant antibacterial activity. Furthermore, the synthesized hydrazone derivatives were found to exhibit significant antidiabetic activity when compared to insulin.
-
[EN] 6H-THIENO`2, 3-B!PYRROLE DERIVATIVES AS ANTAGONISTS OF GONADOTROPIN RELEASING HORMONE (GNRH)<br/>[FR] DERIVES DE 6H-THIENO`2,3-B!PYRROLE EN TANT QU'ANTAGONISTES DE LA GONADOLIBERINE (GNRH)申请人:ASTRAZENECA AB公开号:WO2004018480A1公开(公告)日:2004-03-04The invention relates to a group of novel thieno-pyrrole compounds of Formula (I): wherein: R1, R2, R3, R4 and R5 are as defined in the specification, which are useful as gonadotrophin releasing hormone antagonists. The invention also relates to pharmaceutical formulations of said compounds, methods of treatment using said compounds and to processes for the preparation of said compounds.
-
New Hydrazinothiazole Derivatives of Usnic Acid as Potent Tdp1 Inhibitors作者:Filimonov、Chepanova、Luzina、Zakharenko、Zakharova、Ilina、Dyrkheeva、Kuprushkin、Kolotaev、Khachatryan、Patel、Leung、Chand、Ayine-Tora、Reynisson、Volcho、Salakhutdinov、LavrikDOI:10.3390/molecules24203711日期:——cancer therapy. Combination chemotherapy using Tdp1 inhibitors as a component can potentially improve therapeutic response to many chemotherapeutic regimes. A new set of usnic acid derivatives with hydrazonothiazole pharmacophore moieties were synthesized and evaluated as Tdp1 inhibitors. Most of these compounds were found to be potent inhibitors with IC50 values in the low nanomolar range. The activity
-
Novel Affinity Ligands for Chromatography Using Combinatorial Chemistry作者:Tor Regberg、Charlotta Lindquist、Ake Pilotti、Christel Ellstrom、Lars Fagerstam、Ann Eckersten、Yasuro Shinohara、Steven L. Gallion、Joseph C. HoganDOI:10.2174/138620711795222482日期:2011.5.1Spatially addressable combinatorial libraries were synthesized by solution phase chemistry and screened for binding to human serum albumin. Members of arylidene diamide libraries were among the best hits found, having submicromolar binding affinities. The results were analyzed by the frequency with which particular substituents appeared among the most potent compounds. After immobilization of the ligands either through the oxazolone or the amine substituent, characterization by surface plasmon resonance showed that ibuprofen affected the binding kinetics, but phenylbutazone did not. It is therefore likely that these compounds bind to Site 2 in sub domain IIIA of human serum albumin (HSA).
-
One Pot Synthesis of Micromolar BACE-1 Inhibitors Based on the Dihydropyrimidinone Scaffold and Their Thia and Imino Analogues作者:Jessica Bais、Fabio Benedetti、Federico Berti、Iole Cerminara、Sara Drioli、Maria Funicello、Giorgia Regini、Mattia Vidali、Fulvia FellugaDOI:10.3390/molecules25184152日期:——
A library of dihydropyrimidinones was synthesized via a “one-pot” three component Biginelli reaction using different aldehydes in combination with β-dicarbonyl compounds and urea. Selected 2-thiooxo and 2-imino analogs were also obtained with the Biginelli reaction from thiourea and guanidine hydrochloride, respectively. The products were screened in vitro for their β-secretase inhibitory activity. The majority of the compounds resulted to be active, with IC50 in the range 100 nM–50 μM.
表征谱图
-
氢谱1HNMR
-
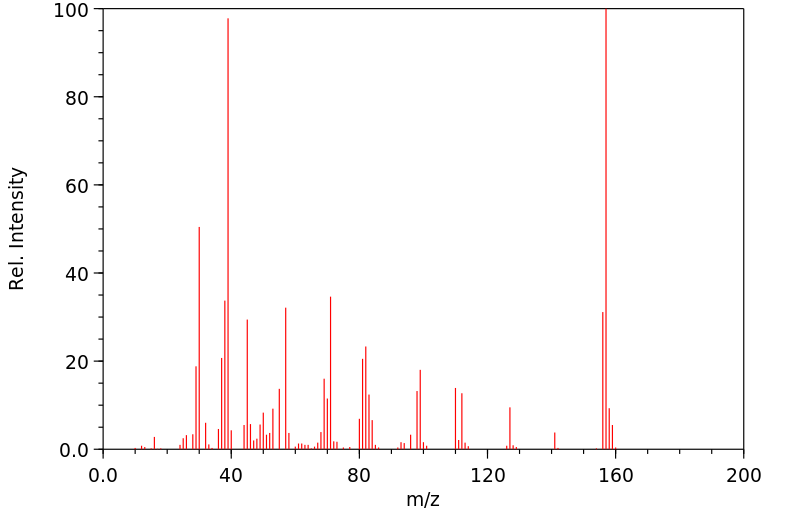
质谱MS
-
碳谱13CNMR
-
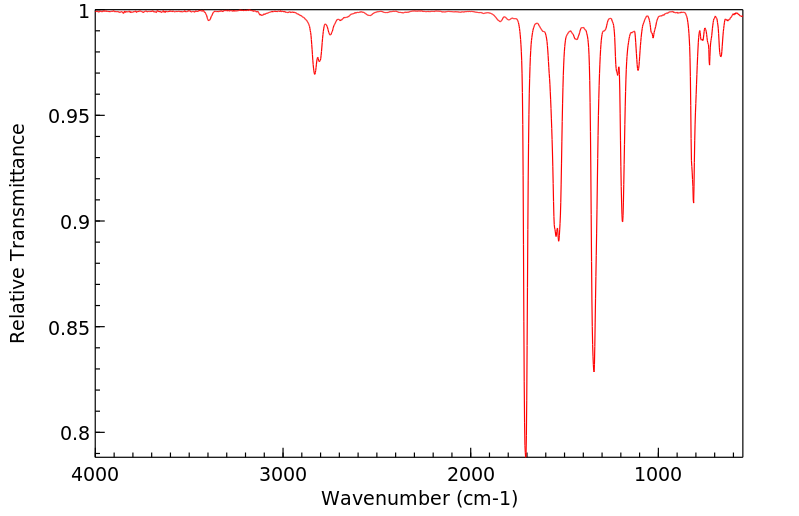
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息