1,3-二氯丙烯 | 542-75-6
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-60 °C
-
沸点:97-112 °C(lit.)
-
密度:1.2
-
闪点:78 °F
-
溶解度:易溶于可溶于氯仿、甲醇
-
暴露限值:ACGIH: TWA 1 ppm (Skin)NIOSH: TWA 1 ppm(5 mg/m3)
-
LogP:2.040
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):1.7
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 1 ppm (5 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:3
-
危险品标志:T
-
安全说明:S36/37,S45,S60,S61
-
危险类别码:R20/21,R36/37/38,R43,R10,R50/53,R25
-
WGK Germany:3
-
海关编码:2903199000
-
危险品运输编号:UN 1992 3/PG 3
-
危险类别:3
-
RTECS号:UC8310000
-
包装等级:II
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过37℃。 - 包装要求密封,避免与空气接触。 - 应与氧化剂和酸类分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
SDS
| 国标编号: | 33528 |
| CAS: | 542-75-6 |
| 中文名称: | 1,3-二氯丙烯 |
| 英文名称: | 1,3-dichloropropene;1,3-dichloropropylene |
| 别 名: | 3-氯丙烯基氯 |
| 分子式: | C 3 H 4 Cl 2 ;ClCHCHCH 2 Cl |
| 分子量: | 111.00 |
| 熔 点: | -84℃ 沸点:108℃ |
| 密 度: | 相对密度(水=1)1.22; |
| 蒸汽压: | 35℃ |
| 溶解性: | 不溶于水,溶于乙醇、乙醚、苯等多数有机溶剂 |
| 稳定性: | 稳定 |
| 外观与性状: | 无色液体,有灯似氯仿的气味 |
| 危险标记: | 7(易燃液体) |
| 用 途: | 用于有机合成和用作防霉剂 |
2.对环境的影响:
一、健康危害
侵入途径:吸入、食入、经皮吸收。 健康危害:吸入、口服或经皮肤吸收对几何有害。对眼睛、皮肤、粘膜和呼吸道有强烈的刺激作用;吸入后可因喉、支气管的痉挛、水肿、炎症,化学性肺炎、肺水肿而致死。中毒症状有烧灼感、咳嗽、喘息、喉炎、气短、头痛、恶心和呕吐。
二、毒理学资料及环境行为
急性毒性:LD50470~710mg/kg(大鼠经口);504mg/kg(兔经皮);LC504650mg/m3,2小时(小鼠吸入) 亚急性和慢性毒性:大鼠吸入50ppm,6小时/天,12周,肝肾肿大,动物存活。 致突变性:微生物致突变:鼠伤鼠寒沙门氏菌100µg/皿。姊妹染色单体交换:仓鼠卵巢900nmol/L。
危险特性:易燃,其蒸气与空气可形成爆炸性混合物。遇明火、高热能引起燃烧爆炸。与氧化剂能发生强烈反应。 燃烧(分解)产物:一氧化碳、二氧化碳、氯化氢、光气。
3.现场应急监测方法:
便携式气相色谱法
4.实验室监测方法:
| 监测方法 | 来源 | 类别 |
| 吹扫捕集-气相色谱法 | 中国环境监测总站 | 水质 |
| 气相色谱法 | 《固体废弃物试验与分析评价手册》中国环境监测总站等译 | 固体废弃物 |
| 色谱-质谱法 | 《水和废水标准检验法》第20版(美) | 水质 |
| 色谱/质谱法 | 美国EPA524.2方法 | 水质 |
5.环境标准:
前苏联 车间空气中有害物质的最高容许浓度 5mg/m3前苏联(1975)水体中有害物质最高允许浓度 0.4mg/L
6.应急处理处置方法:
一、泄漏应急处理
迅速撤离泄漏污染区人员至安全区,并进行隔离,严格限制出入。切断火源。建议应急处理人员戴自给正压式呼吸器,穿消防防护服。尽可能切断泄漏源,防止进入下水道、排洪沟等限制性空间。小量泄漏:用砂土或其它不燃材料吸附或吸收。也可以用不燃性分散剂制成的乳液刷洗,洗液稀释后放入废水系统。大量泄漏:构筑围堤或挖坑收容;用泡沫覆盖,降低蒸气灾害。用防爆泵转移至槽车或专用收集器内,回收或运至废物处理场所处置。
二、防护措施
呼吸系统防护:可能接触其蒸气时,应该佩戴自吸过滤式防毒面具(全面罩)。紧急事态抢救或撤离时,建议佩戴自给式呼吸器。 眼睛防护:呼吸系统防护中已作防护。 身体防护:穿胶布防毒衣。 手防护:戴橡胶手套。 其它:工作现场禁止吸烟、进食和饮水。工作毕,淋浴更衣。注意个人清洁卫生。
三、急救措施
皮肤接触:立即脱去被污染的衣着,用大量流动清水冲洗,至少15分钟。就医。 眼睛接触:立即提起眼睑,用大量流动清水或生理盐水彻底冲洗至少15分钟。就医。 吸入:迅速脱离现场至空气新鲜处。保持呼吸道通畅。如呼吸困难,给输氧。如呼吸停止,立即进行人工呼吸。就医。 食入:误服者用水漱口,给饮牛奶或蛋清。就医。
制备方法与用途
1,3-二氯丙烯为无色有甜味的挥发性液体,不纯时呈白色或琥珀色。它可用作有机合成中间体,并作为除草剂野燕畏和土壤熏蒸剂滴滴混剂中的成分。
化学性质本品为无色有甜味的挥发性液体,在不纯时可呈白色或琥珀色,沸点97~112℃,折射率nD 1.4720,相对密度1.198,冰点23℃,在水中的溶解度为0.1 g/100 g水,可与甲酚、丙酮、苯、四氯化碳和正庚烷相溶。本品主要由顺式和反式1,3-二氯丙烯混合而成,它们的物性如下:顺式1,3-二氯丙烯,沸点104.3℃,折射率nD 1.469,相对密度1.224;反式1,3-二氯丙烯,沸点112℃,折射率nD 1.475,相对密度1.217。
生产方法由丙烯与氯气反应制得。该反应在500~550℃进行,通过分离即可得到产物。在丙烯高温氯化制环氧氯丙烷过程中,副反应生成的混合物中含有3-氯丙烯、1,3-二氯丙烯和1,2-二氯丙烷。在分离这些物质时,在塔顶可得1,3-二氯丙烯,在塔釜则得到滴滴混剂,其中二氯丙烷和二氯丙烯含量达80%以上。每生产1吨环氧氯丙烷,可副产滴滴混剂230kg。
用途1,3-二氯丙烯可用于合成杀虫剂、除草剂等,并且可用作滴滴混剂的成分,具有强力杀线虫作用。一般使用的混合物为50%~60%的1,3-二氯丙烯、25%的1,2-二氯丙烷和其他氯化物,形成的液体为有刺激性氯臭味的黄褐色液体,并且具有腐蚀性和易吸水的特点。
生产方法其制备方法是在500~550℃条件下用丙烯和氯气反应,然后进行分离。在丙烯氯化制备环氧氯丙烷时,副产物包括3-氯丙烯、1,3-二氯丙烯和1,2-二氯丙烷,这些物质同样可以通过分离得到。
类别农药
毒性分级高毒
急性毒性- 大鼠 LD50: 470毫克/公斤
- 小鼠 LD50: 640毫克/公斤
遇明火、高温或氧化剂时较易燃烧,燃烧会产生有毒氯化物烟雾。受热分解会释放有毒光气。
储运特性库房应保持通风低温干燥,并与氧化剂分开存放,不宜久储以防聚合。
灭火剂 职业标准TWA 5毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-氯丙烯 3-chloroprop-1-ene 107-05-1 C3H5Cl 76.5257
反应信息
-
作为反应物:参考文献:名称:1,3-二氯丙醇生产1,3-丙二醇的方法及系统摘要:本发明公开了一种1,3‑二氯丙醇生产1,3‑丙二醇的方法及系统。所述方法包括:将1,3‑二氯丙醇连续输入设置有脱水催化剂的反应装置进行脱水反应,制得1,3‑二氯丙烯;将所述1,3‑二氯丙烯和氢气连续输入设置有加氢催化剂的反应装置中进行加氢反应,制得1,3‑二氯丙烷;以及,使包含所述1,3‑二氯丙烷、水解剂和溶剂的混合反应体系进行水解反应,制得1,3‑丙二醇。本发明以廉价的甘油氯化法制备环氧氯丙烷的中间产物1,3‑二氯丙醇为原料,通过脱水‑加氢‑水解3步制得重要的化工原料1,3‑丙二醇,为甘油制1,3‑丙二醇提供了一条新途径,且该路线条件温和,成本低廉,具有环环保与经济等优势。公开号:CN112479811A
-
作为产物:参考文献:名称:1,3-二氯丙醇生产1,3-丙二醇的方法及系统摘要:本发明公开了一种1,3‑二氯丙醇生产1,3‑丙二醇的方法及系统。所述方法包括:将1,3‑二氯丙醇连续输入设置有脱水催化剂的反应装置进行脱水反应,制得1,3‑二氯丙烯;将所述1,3‑二氯丙烯和氢气连续输入设置有加氢催化剂的反应装置中进行加氢反应,制得1,3‑二氯丙烷;以及,使包含所述1,3‑二氯丙烷、水解剂和溶剂的混合反应体系进行水解反应,制得1,3‑丙二醇。本发明以廉价的甘油氯化法制备环氧氯丙烷的中间产物1,3‑二氯丙醇为原料,通过脱水‑加氢‑水解3步制得重要的化工原料1,3‑丙二醇,为甘油制1,3‑丙二醇提供了一条新途径,且该路线条件温和,成本低廉,具有环环保与经济等优势。公开号:CN112479811A
-
作为试剂:参考文献:名称:Pesticides摘要:化合物式I的化合物##STR1##其中,D代表氢或氰基; X代表卤素; A代表烷基(通常是C.sub.1-C.sub.6烷基); n为0至4; RCOO.代表酸RCO.sub.2 H的残基,该酸或其酯化衍生物与.alpha.-氰基-3-苯氧基苯甲醇或其酯化衍生物反应,产生具有杀虫性能的.alpha.-氰基-3-苯氧基苯甲酸酯。公开号:US04847438A1
文献信息
-
[EN] CONJUGATES FOR TREATING DISEASES<br/>[FR] CONJUGUÉS POUR LE TRAITEMENT DE MALADIES申请人:ENDOCYTE INC公开号:WO2016148674A1公开(公告)日:2016-09-22The present disclosure relates to pyrrolobenzodiazepine (PBD) prodrugs and conjugates thereof. The present disclosure also relates to pharmaceutical compositions of the conjugates described herein, methods of making and methods of using the same.
-
[EN] PYRAZOLE COMPOUNDS AND USE THEREOF IN NOXIOUS ARTHROPOD PESTS CONTROLLING COMPOSITION<br/>[FR] COMPOSES DERIVES DU PYRAZOLE ET LEUR UTILISATION DANS UNE COMPOSITION DE LUTTE CONTRE LES ARTHROPODES NUISIBLES申请人:SUMITOMO CHEMICAL CO公开号:WO2005075433A1公开(公告)日:2005-08-18The present invention provides a pyrazole compound of formula (a): ;a noxious arthropod pests controlling composition containing the compound shown by the formula (a) as an active ingredient; and a method for controlling noxious arthropod pests comprising applying an effective amount of the compound shown by the formula (a).本发明提供了一种式(a)的吡唑化合物;一种含有所述式(a)化合物作为活性成分的有害节肢动物害虫控制组合物;以及一种控制有害节肢动物害虫的方法,包括施用所述式(a)化合物的有效量。
-
A Mild and Convenient Barbier-Type Allylation of Aldehydes to Homoallylic Alcohols via Iodide Ion Promoted Stannylation of Allylic Bromides and Chlorides with Tin(II) Chloride作者:Toshiro Imai、Shinya NishidaDOI:10.1055/s-1993-25871日期:——Barbier-type allylation of aldehydes with allylic bromides and tin(II) chloride dihydrate is largely accelerated by adding stoichiometric or substoichiometric amounts of sodium iodide. This method has some merits such as lower temperature, shorter reaction time and/or more choices of solvents for the reaction. Moreover, the activation by the iodide ion enables the use of relatively unreactive allylic chlorides of various structural types (e.g., 3-chloro-2-chloromethylpropene as an isobutene dianion equivalent) and, thus, expands synthetic applicability of this reaction. The major role of the iodide salt is attributed to the in situ generation of the corresponding allylic iodide.
-
A simple, stereoselective, room-temperature synthesis of cis vinyloxiranes and trans 1-phenyl-1,3-butadiene作者:Jacques Auge、Serge DavidDOI:10.1016/s0040-4039(00)88249-0日期:1983.1The organotin reagent from 1-chloro-3-iodoprop-1-ene and SnCl2 in dimethylformamide reacted with aldehydes by ite chlorine-substituted carbon atom. Treatment with NaOMe then gave vinyloxiranes with good stereo-selectivity. benzaldehyde and 1-bromo-3-iodoprop-1-ene in the presence of two equivalents of SnCl2 gave exclusively -1-phenyl-1,3-butadiene.
-
SYNTHESIS OF β, γ-UNSATURATE CARBOXYLIC ACID DERIVATIVES BY THE NOVEL Ni (CO)<sub>4</sub>-INDUCED RING-OPENING CARBONYLATION REACTION OF 1,1-DIBROMO-2-CHLOROCYCLOPROPANES作者:Toshikazu Hirao、Shinichiro Nagata、Toshio AgawaDOI:10.1246/cl.1985.1625日期:1985.11.51,1-Dibromo-2-chlorocyclopropanes underwent the Ni (CO)4-induced ring-opening carbonylation reaction with alcohol or amine giving the β, γ,-unsaturated carboxylic acid and dicarboxylic acid derivatives. Use of N,N-dimethyltrimethylsilylamine as an initial nucleophile in the presence of benzaldehyde led to a dienecarboxamide presumably via codensation of the nickel enolate intermediate.
表征谱图
-
氢谱1HNMR
-
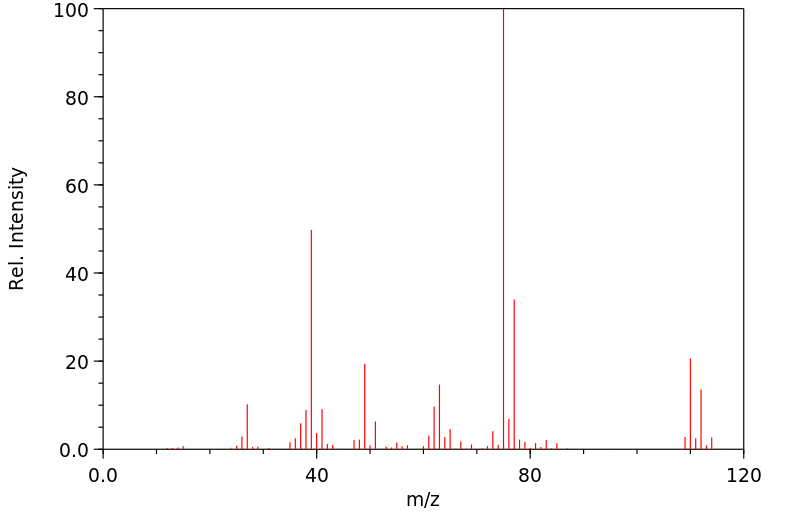
质谱MS
-
碳谱13CNMR
-
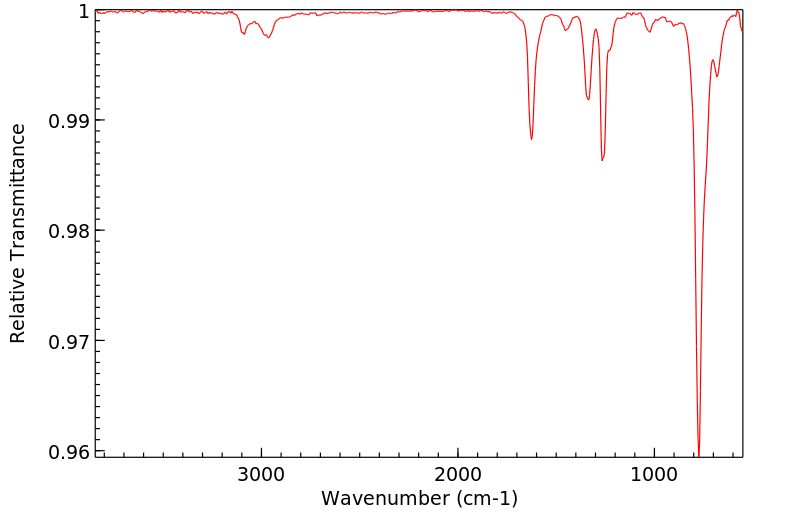
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息