α,α-二溴甲苯 | 618-31-5
中文名称
α,α-二溴甲苯
中文别名
a,a-二溴甲苯;亚苄基二溴;二溴甲苯
英文名称
benzylidene dibromide
英文别名
benzal bromide;(Dibromomethyl)benzene;dibromomethylbenzene
CAS
618-31-5
化学式
C7H6Br2
mdl
——
分子量
249.933
InChiKey
VCJZTATVUDMNLU-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:111℃
-
沸点:156 °C23 mm Hg(lit.)
-
密度:1.51 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):3.4
-
重原子数:9
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.14
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
危险等级:6.1(a)
-
安全说明:S24/25
-
危险类别码:R23/24,R33
-
海关编码:2903999090
-
包装等级:II
-
危险类别:6.1(a)
-
WGK Germany:3
-
危险品运输编号:UN 2927 6.1/PG 2
-
危险品标志:T
-
储存条件:密封储存,应存放在阴凉、干燥的仓库中。
SDS
| Name: | Alpha Alpha-Dibromotoluene 97% Material Safety Data Sheet |
| Synonym: | Benzal Bromide |
| CAS: | 618-31-5 |
Synonym:Benzal Bromide
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 618-31-5 | Benzal Bromide | 97 | 210-543-7 |
Risk Phrases: 23/24 33
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Toxic by inhalation and in contact with skin. Danger of cumulative effects.Lachrymator (substance which increases the flow of tears).The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation and possible burns. Lachrymator (substance which increases the flow of tears). May cause chemical conjunctivitis and corneal damage.
Skin:
May cause severe irritation and possible burns.
Ingestion:
May cause severe gastrointestinal tract irritation with nausea, vomiting and possible burns. The toxicological properties of this substance have not been fully investigated.
Inhalation:
The toxicological properties of this substance have not been fully investigated. May cause severe respiratory tract irritation and possible burns. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid. Do NOT allow victim to rub eyes or keep eyes closed.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Containers may explode when heated.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Cool containers with flooding quantities of water until well after fire is out. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Remove all sources of ignition. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation. Wash clothing before reuse.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 618-31-5: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear very slight orange to brown
Odor: toluene-like odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 156 deg C @ 23.00mm Hg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: 110 deg C ( 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.5100g/cm3
Molecular Formula: C7H6Br2
Molecular Weight: 249.93
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat.
Incompatibilities with Other Materials:
Strong bases, strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen bromide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 618-31-5 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Benzal Bromide - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.*
Hazard Class: 6.1
UN Number: 2810
Packing Group: III
IMO
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing Group: II
RID/ADR
Shipping Name: TOXIC LIQUID, ORGANIC, N.O.S.
Hazard Class: 6.1
UN Number: 2810
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: T
Risk Phrases:
R 23/24 Toxic by inhalation and in contact with
skin.
R 33 Danger of cumulative effects.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 618-31-5: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 618-31-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 618-31-5 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 三溴甲基苯 tribromomethylbenzene 2489-03-4 C7H5Br3 328.829 溴甲苯 benzyl bromide 100-39-0 C7H7Br 171.037 3-氯代亚苄基二溴 1-chloro-4-(dibromomethyl)benzene 62037-06-3 C7H5Br2Cl 284.378 1-氯-2-(二溴甲基)苯 2-chlorobenzal bromide 67013-54-1 C7H5Br2Cl 284.378 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 三溴甲基苯 tribromomethylbenzene 2489-03-4 C7H5Br3 328.829 溴甲苯 benzyl bromide 100-39-0 C7H7Br 171.037 3-氯代亚苄基二溴 1-chloro-4-(dibromomethyl)benzene 62037-06-3 C7H5Br2Cl 284.378 1-氯-2-(二溴甲基)苯 2-chlorobenzal bromide 67013-54-1 C7H5Br2Cl 284.378
反应信息
-
作为反应物:描述:参考文献:名称:卤代烷通过可见光介导的脱卤作用。摘要:可以通过简单的全卤化/脱卤序列以通常高收率进行活化的CH键的净选择性溴化和氯化。光化学还原不需要光催化剂,而是依赖于底物和还原剂的电子给体-受体复合物的形成,或者自动光催化。尽管有任何明显的光子吸收,但仍会发生一些反应,这是涉及胺类的其他光化学反应的警惕故事。机械实验为这种观察提供了解释。DOI:10.1021/acs.orglett.9b03848
-
作为产物:参考文献:名称:Kondratenko,N.V. et al., Journal of Organic Chemistry USSR (English Translation), 1970, vol. 6, p. 1436 - 1439摘要:DOI:
-
作为试剂:描述:参考文献:名称:Homolytic addition of benzylidene bromide to trimethylvinylsilane and 1-hexene摘要:Benzylidene bromide reacts with unsaturated compounds CH2=CHR (R = Bu, SiMe(3)) in the presence of benzoyl peroxide to give PhCHBrCH(2)CHRBr adducts.DOI:10.1007/bf00698261
文献信息
-
Palladium(0) Complex-Catalyzed Debrominative Coupling of (Tribromomethyl)- and (Dibromomethyl)benzenes to Diarylacetylenes and 1,2-Diarylethenes作者:Shuntaro Mataka、Guo-Bin Liu、Masashi TashiroDOI:10.1055/s-1995-3878日期:1995.2Palladium(0)-triphenylphosphine complex-catalyzed debrominative coupling reaction of (tribromomethyl)benzenes gave diarylacetylenes or a mixture of (E)- and (Z)-α,β-dibromostilbenes depending upon the substrate and the solvent being used. On the other hand, (dibromomethyl)benzenes afforded (E)-stilbenes selectively under the same reaction conditions.
-
Glutamate aggrecanase inhibitors
-
Substituted N, N-disubstituted diamino compounds useful for inhibiting cholesteryl ester transfer protein activity申请人:——公开号:US20020120011A1公开(公告)日:2002-08-29The invention relates to substituted polycyclic aryl and heteroaryl tertiary-heteroalkylamine compounds useful as inhibitors of cholesteryl ester transfer protein (CETP; plasma lipid transfer protein-I) and compounds, compositions and methods for treating atherosclerosis and other coronary artery diseases. Preferred tertiary-heteroalkylamine compounds are substituted N,N-disubstituted diamines. A preferred specific N,N-disubstituted diamine is the compound: 1
-
N-Heterocyclic Carbene-Protected Ag Nanoparticles Immobilized on Polyacrylonitrile Fiber as Efficient Catalysts for a Three-Component Coupling Reaction作者:Jian Cao、Hongqi TianDOI:10.1002/asia.201800154日期:2018.6.18report the preparation and characterization of N‐heterocyclic carbene (NHC)‐stabilized silver NPs supported on polyacrylonitrile fiber (PANF). As a result, Ag loadings of up to 8 % and particle sizes of 11.0±3.2 nm were achieved. This novel nanocomposite catalyst demonstrated high activity in addition to excellent stability and reusability in the three‐component reaction between alkynes, haloalkanes金属纳米颗粒(NPs)通常通过引入封端剂或其在载体上的分布来稳定。在此背景下,我们报告了聚丙烯腈纤维(PANF)上负载的N杂环卡宾(NHC)稳定的银NP的制备和表征。结果,实现了高达8%的Ag负载和11.0±3.2nm的粒径。除了在炔烃,卤代烷烃和胺的三组分反应中具有出色的稳定性和可重复使用性之外,这种新型的纳米复合催化剂还具有很高的活性。在该系统中,通过支持作用和配体作用使AgNP稳定。独特的NHC保护的AgNP结构和PANF支持在C sp的去质子化中发挥协同作用-H键,周转数高达3500。该催化剂成功地循环使用了八次,活性没有明显损失,并且AgNPs没有明显的聚集。此外,使用PANF-NHC @ Ag作为催化剂的流动系统的实施可产生57 mmol h -1的有效生产率。
-
Phosphorus(V)-catalyzed deoxydichlorination reactions of aldehydes作者:Jie An、Xiaoping Tang、Joshua Moore、William Lewis、Ross M. DentonDOI:10.1016/j.tet.2013.07.100日期:2013.10phosphorus(V)-catalysis manifold in which phosphine oxide turnover is achieved using oxalyl chloride as a consumable reagent. The new method is applicable to a range of aldehydes and, in combination with palladium-catalyzed reductive dimerization, gives rise to a new catalytic approach to the synthesis of stilbenes and a short formal synthesis of resveratrol.
表征谱图
-
氢谱1HNMR
-
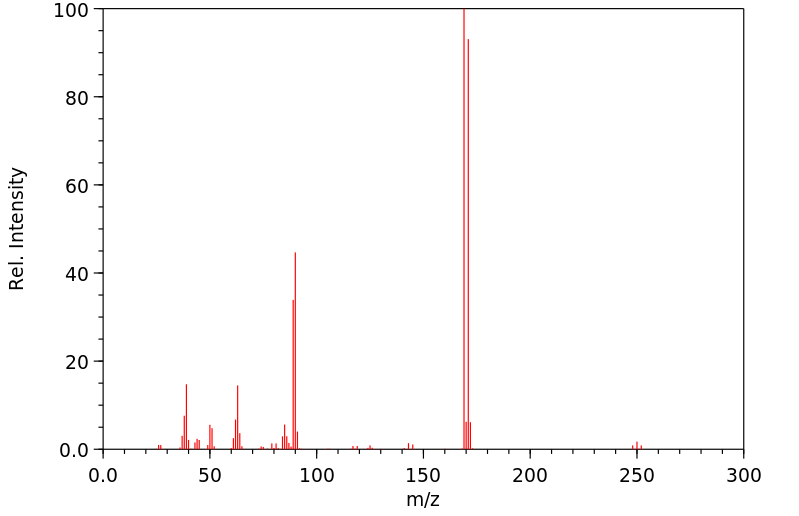
质谱MS
-
碳谱13CNMR
-
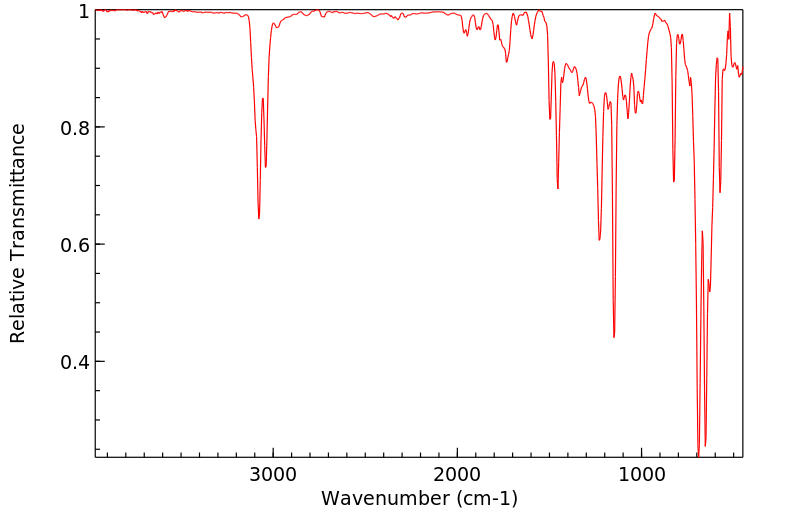
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫